Histomoniasis is caused by Histomonas meleagridis, a flagellated protozoan parasite. It causes systemic infection in turkeys and induces characteristic lesions in ceca and liver. Although the bursa of Fabricius is not a target organ for H. meleagridis, it is susceptible to infection due to its anatomical location adjacent to the hindgut. While H. meleagridis causes gross lesions in the liver and ceca, microscopic examination is required for lesion assessment within the bursa of Fabricius. Therefore, the pathologic effects of H. meleagridis in the bursa of Fabricius is underreported. This study evaluates histologic changes in ceca and the bursa of Fabricius.
Vijay Durairaj1, Mary Drozd2, Ryan Vander Veen1
1 Huvepharma, Inc., Lincoln, NE 68528
2 University of Nebraska-Lincoln, Lincoln, NE 68583-0907
Poults (n=14) were challenged by intra-cloacal infection wild-type H. meleagridis field isolates. Placebo controls (n=5) were commingled with the challenged poults. Poults were raised in an isolator and provided ad libitum feed and water. Poults were necropsied nine days post-challenge. All challenged poults and one of the commingled placebo birds had distinctive cecal lesions. On histology, cecal lesions ranging from mild edema, histiocytic and heterophilic inflammation, to necroulcerative and fibrinonecrotizing typhlitis with Histomonas trophozoites were observed in the challenged poults. Microscopic lesions in the bursa of Fabricius of challenged poults ranged from mild to moderate lymphoid hyperplasia, bursitis, heterophilic and histiocytic infiltration accompanied by decreased lymphoid cell density and presence of Histomonas trophozoites. The severity of cecal lesions positively correlated with histologic changes in the bursa of Fabricius.
Abbreviations: CGL – Cecal gross lesions; H & E stain – Hematoxylin and eosin stain; HMA – Histomonas meleagridis isolate A; HMB – Histomonas meleagridis isolate B; LGL – Liver gross lesions; M199 – Medium 199; MCLS – Microscopic cecal lesion score; MDM – Modified Dwyer’s media; PAS – Periodic acid-Schiff’s stain.
Introduction
Histomoniasis is a deadly disease in turkeys causing up to 100% mortality. It is caused by Histomonas meleagridis, a protozoan parasite. The eggs of Heterakis gallinarum can harbor H. meleagridis and transmit the disease. Histomoniasis field outbreaks occur either due to direct transmission of H. meleagridis or through indirect transmission through vector and reservoirs such as H. gallinarum and earthworms. Mechanical vectors such as flies and insects can also transmit the disease. Direct contact such as cloacal drinking or contaminated equipment favors the lateral transmission of the disease within the flock. The cloacal movements facilitate H. meleagridis migration to ceca and the bursa of Fabricius. Ceca infection results in extensive protozoal infiltration through the intestinal wall, heterophilic and granulomatous inflammation, and necrosis which results in hemorrhage and characteristic cecal cores. Hypotheses for the most prevalent method of systemic dissemination include hematogenous spread of H. meleagridis from the ceca to the liver and other visceral organs and direct spread through intestinal rupture and peritoneal seeding.
Gross liver lesions may vary from pin-point white necrotic foci to pale white circumscribed periphery with dark red centers resembling bulls-eyes, and pale white necrotic foci with round depressions. In addition to hepatitis and typhlitis, histomoniasis also causes lesions in kidneys, lungs, spleen, pancreas, proventriculus and the bursa of Fabricius. The systemic nature of disease and anatomical location of the bursa of Fabricius provides an easily accessible entry for H. meleagridis. Lymphoid depletion in the bursa of Fabricius has been documented in histomoniasis outbreaks in commercial chickens. In addition to gross lesions in the ceca and liver, histology reveals infection of additional organs including the bursa of Fabricius. The objective of this study was to conduct microscopic evaluation of the ceca and bursa in turkeys challenged with wild-type field isolates of H. meleagridis and further clarify bursal pathology associated with H. meleagridis.
Materials and methods
Case history
Two wild-type field isolates of H. meleagridis (HMA and HMB) were used in this study. HMA and HMB were isolated from turkey flocks experiencing histomoniasis outbreaks in Midwest and Southeastern USA, respectively. Histomonads were cultured in Modified Dwyer’s Media (MDM) at 40 °C with 0.8% (wt/vol) rice powder, 0.35% (wt/vol) sodium bicarbonate (Sigma-Aldrich, St. Louis, MO 63103) and 5% horse serum (HyClone, South Logan, UT 84321) in Medium 199 with Hank’s balanced salt solution (Sigma-Aldrich, St. Louis, MO 63103). One ml of each H. meleagridis culture was sub-cultured in 10 ml of MDM and incubated anaerobically at 40 °C. The growth of histomonads was monitored periodically by examining the culture flask under inverted microscope. Enumeration of the histomonads was performed using a hemocytometer.
Experimental study
Nineteen day-of-hatch turkey poults (Nicholas Turkeys) were obtained from a local hatchery. At fifteen days-of-age all poults were assigned individual ID numbers and placed in an isolator. Seven poults were challenged either with 1.9×105 HMA histomonads/ 0.5 ml dose or with 6.24 x104 HMB histomonads/ 0.5 ml by intra-cloacal administration. Five poults received MDM as placebo by intra-cloacal inoculation and were co-mingled with challenged poults. All poults were provided with ad libitum unmedicated feed and water.
Gross lesions and sample collection
Poults were necropsied and classified as positive or negative based on gross lesions in the liver at nine days post-challenge. Histology samples were collected in 10% neutral buffered formalin. Ceca and bursa samples were collected from all 5 comingled placebo birds. Eleven cecal samples and 10 bursal samples (one sample was missed during the collection process) were collected from challenged poults.
Histopathology
Sections of tissue fixed in 10% neutral buffered formalin were routinely processed overnight on a Leica Peloris II tissue processor. Paraffin blocks were cut to 5 µm thickness using a Leica RM2255 microtome, stained with hematoxylin and eosin on a Leica ST5020 H&E stainer, and cover-slipped with a Leica CV5030 coverslip. Three-five-micron thick paraffin embedded sections were deparaffinized in Xylene and rinsed. Slides were immersed in 0.5% Periodic Acid solution for 5 minutes. Slides were rinsed in 4 changes of distilled water and immersed in Schiff Reagent for 15 minutes. Slides then rinsed under tap water for 10 minutes before immersed in a 0.2% aqueous Fast green counter stain. Slides were similarly rinsed in tap water for 10 minutes and oven-dried for 10 minutes before manually cover-slipped.
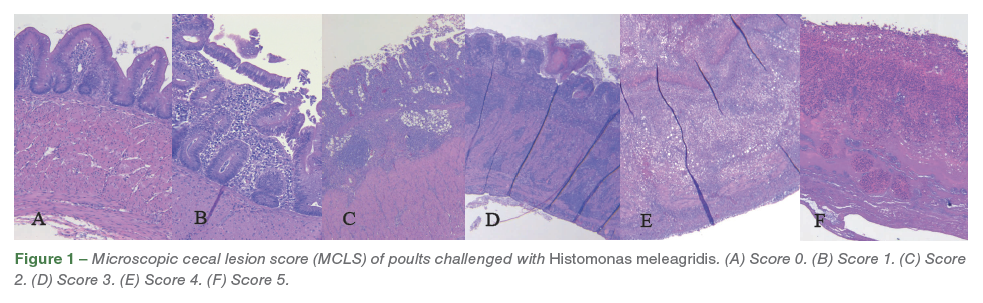
A veterinary anatomic pathologist was blinded for this study and performed all histological evaluations. On histopathological evaluation, ceca was graded from 0-5 based on microscopic cecal lesion score (MCLS) as follows. Score 0 = normal ceca (Figure 1A); Score 1 = mild to moderate erosion of mucosa, expansion of lamina propria with histiocytes, few trophozoites, with intact mucosa and submucosa (Figure 1B); Score 2 = moderate to abundant expansion of lamina propria with moderate to abundant histiocytes, trophozoites, mucosa and submucosa are moderately infiltrated, mucosa is significantly ulcerated with intact tunica muscularis (Figure 1C); Score 3 = lamina propria and mucosa is extensively effaced by histiocytes and trophozoites, submucosa is moderately infiltrated and effaced by inflammation and organisms, tunica muscularis is infiltrated by inflammation/ protozoa but architecture can be clearly discerned (Figure 1D); Score 4 = lamina propria, mucosa, submucosa is severely to completely effaced by histiocytes and trophozoites, tunica muscularis is severely infiltrated by inflammation/protozoa with extensive loss of muscle architecture (Figure 1E); Score 5 = full transmural necrosis (Figure 1F).
Results
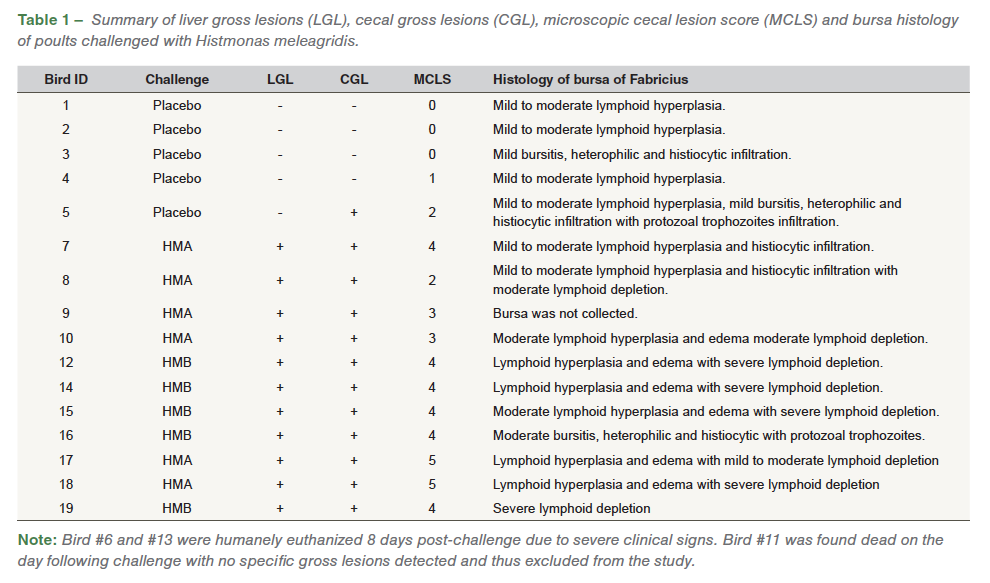
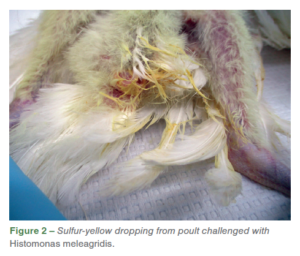 Both wild-type isolates HMA and HMB resulted in 100% incidences associated with gross lesions in ceca and liver for H. meleagridis. Distinctive gross lesions in ceca and/ or liverwere considered as positive for histomoniasis. On the day following challenge, one of the challenged poults (ID# 11) was found dead with no lesions detected and was subsequently excluded from the study. Eight days post-challenge, two birds (ID# 6 and 13) exhibited severe clinical signs such as dullness and depression, and were euthanized due to humane reasons and samples were not collected for histology. In addition to the clinical signs, some of the challenged poults also had sulfur-yellow droppings (Figure 2). Poults challenged with HMA and HMB had distinctive liver and cecal lesions characteristic of H. meleagridis. One of the comingled poults (ID# 5) also had severe gross lesions in ceca and liver.
Both wild-type isolates HMA and HMB resulted in 100% incidences associated with gross lesions in ceca and liver for H. meleagridis. Distinctive gross lesions in ceca and/ or liverwere considered as positive for histomoniasis. On the day following challenge, one of the challenged poults (ID# 11) was found dead with no lesions detected and was subsequently excluded from the study. Eight days post-challenge, two birds (ID# 6 and 13) exhibited severe clinical signs such as dullness and depression, and were euthanized due to humane reasons and samples were not collected for histology. In addition to the clinical signs, some of the challenged poults also had sulfur-yellow droppings (Figure 2). Poults challenged with HMA and HMB had distinctive liver and cecal lesions characteristic of H. meleagridis. One of the comingled poults (ID# 5) also had severe gross lesions in ceca and liver.
The liver gross lesions (LGL) ranged from few pale necrotic foci to multiple, coalescing necrotic foci with or without extensive liver discoloration (Figures 3. A, B, C).
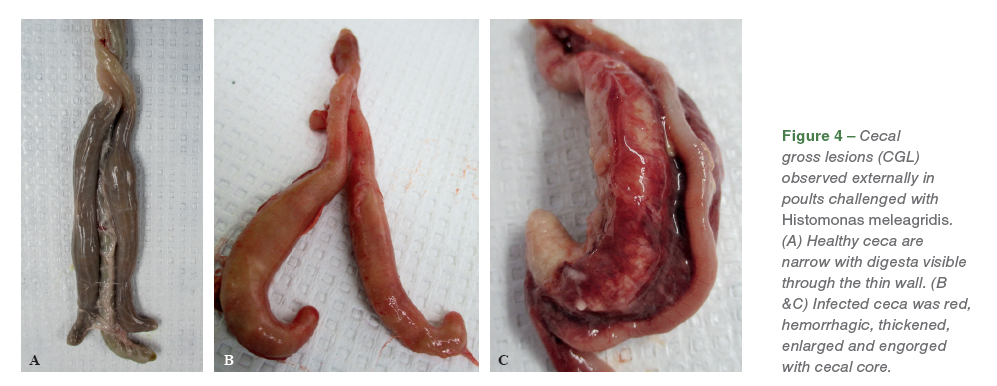
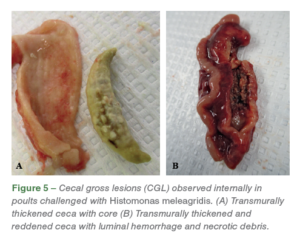
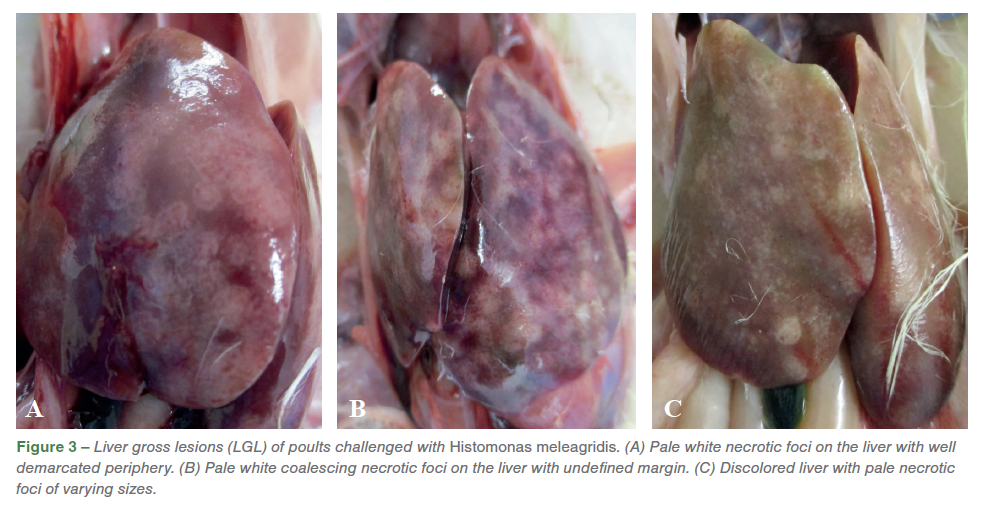 The necrotic foci on the liver varied from diffuse lesions with undefined periphery to circumscribed lesions. On external examination, healthy ceca are narrow with digesta visible through the thin wall (Figure 4. A), while the infected ceca was red, hemorrhagic, thickened, enlarged and engorged with cecal core. (Figures 4. B, C). On transection, the affected ceca were transmurally thickened, reddened, and contained luminal hemorrhage and necrotic debris (Figure 5. A, B).
The necrotic foci on the liver varied from diffuse lesions with undefined periphery to circumscribed lesions. On external examination, healthy ceca are narrow with digesta visible through the thin wall (Figure 4. A), while the infected ceca was red, hemorrhagic, thickened, enlarged and engorged with cecal core. (Figures 4. B, C). On transection, the affected ceca were transmurally thickened, reddened, and contained luminal hemorrhage and necrotic debris (Figure 5. A, B).
On histology, the cecal mucosa was frequently eroded, ulcerated, and diffusely and abundantly infiltrated by Histomonas trophozoites, heterophilic and pyogranulomatous inflammation, edema and fibrin. Lamina propria were abundantly expanded by similar inflammation and hemorrhage with villus fusion and goblet cell hyperplasia. The underlying submucosa, muscularis mucosa, and serosa were abundantly to markedly infiltrated and effaced by similar inflammation, infectious organisms, neovascularization, fibrin, edema and cellular debris (Figure 1). The coelomic mesentery, fat and abdominal air sacs were thickened by histiocytic inflammation with fewer heterophils, fibrosis, edema, and congestion. The abdominal air sac epithelium was thickened by epithelial hyperplasia and infiltration by heterophils and histiocytes. Histopathological evaluation of ceca revealed necroulcerative and fibrinonecrotizing typhlitis with abundant protozoal trophozoites. All of the challenged poults had a microscopic cecal lesion score (MCLS) of ≥2 (11/11), while one of the commingled bird (ID# 5) had a MCLS of 2 (1/5) and other poults had MCLS of ≤1.
Bursal lymphoid follicles had decreased lymphoid cell density and mildly increased numbers of histiocytes (Figure 6). Bursa was graded and assessed as a percent of lymphoid follicles with increased (mild, moderate, severe) central pallor due to decreased cell density in sections containing more than 30 follicles (Figure 6. A, B). Pale eosinophilic, protozoal trophozoites with a 3-5 μm diameter clear zone surrounding the organism was noticed in the bursa of Fabricius (Figure 6. C, D, E, F).
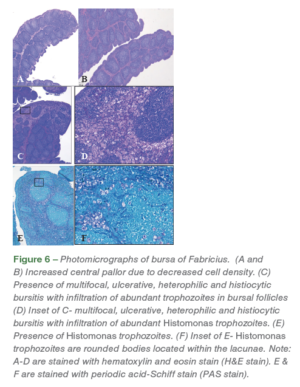 Heterophilic and histiocytic inflammation along with infiltration of inflammatory cells was also noticed in the bursa. Multifocal segments of the bursal epithelium were variably eroded by inflammation and infectious organisms characterized by thinning and flattening of columnar epithelial cells and squamous metaplasia. Lymphoid depletion was assessed without concurrent inflammatory changes. Out of the ten bursal samples analyzed from challenged poults, mild to moderate lymphoid hyperplasia (10/10), edema (6/10), heterophilic and histiocytic infiltration (4/10), moderate (3/10) and severe (5/10) lymphoid depletion, and presence of Histomonas trophozoites (1/10) was noticed. In the commingled placebo poults, mild to moderate lymphoid hyperplasia (4/5), mild bursitis, histiocytic and heterophilic infiltration (2/5), and presence of Histomonas trophozoites (1/5) was noticed (Table 1).
Heterophilic and histiocytic inflammation along with infiltration of inflammatory cells was also noticed in the bursa. Multifocal segments of the bursal epithelium were variably eroded by inflammation and infectious organisms characterized by thinning and flattening of columnar epithelial cells and squamous metaplasia. Lymphoid depletion was assessed without concurrent inflammatory changes. Out of the ten bursal samples analyzed from challenged poults, mild to moderate lymphoid hyperplasia (10/10), edema (6/10), heterophilic and histiocytic infiltration (4/10), moderate (3/10) and severe (5/10) lymphoid depletion, and presence of Histomonas trophozoites (1/10) was noticed. In the commingled placebo poults, mild to moderate lymphoid hyperplasia (4/5), mild bursitis, histiocytic and heterophilic infiltration (2/5), and presence of Histomonas trophozoites (1/5) was noticed (Table 1).
Discussion
Both wild-type H. meleagridis isolates HMA and HMB resulted in distinctive gross lesions in both liver and ceca in challenged poults. The histologic lesions noticed in the bursa of Fabricius were associated with H. meleagridis and supported by presence of Histomonas trophozoites in the bursa of Fabricius. Any compromise to the bursa of Fabricius associated with lymphoid depletion, as observed in most of the challenged birds, would adversely affect the humoral immune response of the host. One of the commingled poults had cecal lesions indicative of lateral transmission of the disease, which was confirmed by the presence of Histomonas trophozoites in ceca as well as the bursa of Fabricius. The histomorphometric changes in the bursa of Fabricius, along with the presence of Histomonas trophozoites in a co-mingled bird, clearly demonstrates the lateral transmission of the disease between challenged and control poults. Contact between these birds, such as cloacal drinking, might have facilitated disease transmission. This agrees with the lateral disease transmission that was previously explained. Mild to moderate lymphoid hyperplasia in the bursa of Fabricius was observed in poults with MCLS of ≥2, while moderate to severe lymphoid depletion in the bursa of Fabricius was observed in poults with MCLS ≥3. This indicates that compromised cecal integrity favors the entry of H. meleagridis into the bursa of Fabricius. Both wild-type field isolates induced positively correlated histological effects in both ceca and the bursa of Fabricius. Further work is needed to understand the impact of H. meleagridis on the bursa of Fabricius and its effect on the humoral immune system.
Acknowledgements
We thank Dr. Elle Chadwick, Deborrah Higuchi, Laura Trygstad, Jess Houchen, Nerissa Ahern and April Cruse for their valuable contributions to this study.
References
1. Hess M, McDougald LR. Histomoniasis. In: Swayne D, Boulianne M, Logue C, McDougald L, Nair V, Suarez D, deWit S, Grimes T, Johnson D, Kromm M, et al., editors. Diseases of poultry. 14th ed. Ames (IA): Wiley-Blackwell. p. 1223–1230; 2020.
2. Cushman S. The production of turkeys. Bulletin 25, Agricultural Experiment Station, Rhode Island College of Agriculture and Mechanical Arts, Kingston, RI. pp. 89–123; 1893.
3. Gibbs BJ. Occurrence of protozoan parasite Histomonas meleagridis in adults and eggs of cecal worm Heterakis gallinae. J. Protozool. 9:288–293; 1962.
4. Hu J, McDougald LR. Direct lateral transmission of Histomonas meleagridis in turkeys. Avian Dis. 47:489–492; 2003.
5. Lund EE, Wehr EE, Ellis DJ. Earthworm transmission of heterakis and histomonas to turkeys and chickens. J. Parasitol. 52:899–902; 1966.
6. Kemp RL, Franson JC. Transmission of Histomonas meleagridis to domestic fowl by means of earthworms recovered from pheasant yard soil. Avian Dis. 19:741–744; 1975.
7. Hu J, Fuller L, McDougald LR. Infection of turkeys with Histomonas meleagridis by the cloacal drop method. Avian Dis. 48:746–750; 2004.
8. Hu J, McDougald LR. Direct lateral transmission of Histomonas meleagridis in turkeys. Avian Dis. 47:489–492; 2003.
9. Armstrong PL, McDougald LR. The infection of turkey poults with Histomonas meleagridis by contact with infected birds or contaminated cages. Avian Dis. 55:48-50; 2011.
10. Sorvari R, Naukkarinen A, Sorvari TE. Anal sucking-like movements in chicken and chick-embryo followed by transportation of environmental material to bursa of Fabricius, ceca and cecal tonsils. Poult. Sci. 56:1426–1429; 1977.
11. Durairaj V, Drozd M, Lin G, De Keyser K, Veen RV. Pathological investigation of a histomoniasis outbreak in turkeys. EC Vet. Sci. 7.1:78-82; 2022.
12. Clarkson MJ. The blood supply of the liver of the turkey and the anatomy of the biliary tract with reference to infection with Histomonas meleagridis. Res. Vet. Sci. 2:259–264; 1961.
13. Singh A, Weissenbock H, Hess M. Histomonas meleagridis: immunohistochemical localization of parasitic cells in formalin-fixed, paraffin-embedded tissue sections of experimentally infected turkeys demonstrated the wide spread of the parasite in its host. Exp. Parsitol. 118:505–513; 2008.
14. Senties-Cue G, Chin RP, and Shivaprasad HL. Systemic histomoniasis associated with high mortality and unusual lesions in the bursa of fabricius, kidneys, and lungs in commercial turkeys. Avian Dis. 53:231–238; 2009.
15. Marx DJ. Turkey bursa of Fabricius infected with Histomonas meleagridis. J. Protozool. 20:519; 1973.
16. Hauck R, Stoute S, Chin RP, Sentíes-Cué CG, Shivaprasad HL. Retrospective Study of Histomoniasis (Blackhead) in California Turkey Flocks, 2000-2014. Avian Dis. 62:94-100; 2018.
17. Cortes PL, Chin RP, Bland MC, Crespo R, Shivaprasad HL. Histomoniasis in the bursa of Fabricius of chickens. Avian Dis. 48:711–715; 2004.
18. McDougald LR, Hu J. Blackhead disease (Histomonas meleagridis) aggravated in broiler chickens by concurrent infection with cecal coccidiosis (Eimeria tenella). Avian Dis. 45:307–312; 2001.
19. Durairaj V, Clark S, Barber E. Vanderveen R. Concurrent infection of Histomonas meleagridis and Pentatrichomonas hominis in a blackhead disease outbreak in turkeys. Avian Dis. 67:124-129; 2023.
20. Hauck R, Armstrong PL, McDougald LR. Histomonas meleagridis (Protozoa: Trichomonadidae): analysis of growth requirements in vitro. J. Parasitol. 96:1–7; 2010.
21. Tyzzer EE, Collier J. Induced and natural transmission of blackhead in the absence of Heterakis. J. Infect. Dis. 37:265–276; 1925.
22. Durairaj V, Vander Veen R. Establishment of Histomonas meleagridis challenge model in turkeys: An industry perspective. IAHJ. 10: 32–37; 2023.
23. Durairaj V, Linnemann E, Icard AH, Williams SM, Sellers HS, Mundt E. An in vivo experimental model to determine antigenic variations among infectious bursal disease viruses. Avian Pathol. 42:309-315; 2013