
This brief review focuses on the types of fibre that the nutritionist should be considering and how a phase feeding strategy should not only take into account the changing needs of the bird as it ages, but also the changing needs of the microbiota if good intestinal health is to be maintained.
In a Google search the term “Gut health” appeared in 19,400 pdf articles published from 1st Jan 2020 to the 3rd October 2024 and the AI generated summary suggested the main factors influencing gut health included:
- Diet – Nutrients and additives
- Water quality
- Environmental temperature, humidity
- Management
- Pathogen exposure
- Immune system status.
Clearly this topic is of great interest and all the factors above are important and need consideration. This is especially the case in regions where antimicrobial growth promoters (AGP’s) have either been banned or removed from feeds on a voluntary basis. In such places, the incidence and severity of intestinal disorders and disease has markedly increased, hence the desire to manage gut health has intensified. Animal husbandry (which is involved in factors 2-5 above) plays by far the greater role in determining the likelihood of such disorders, but diet also has an influence. Most dietary interventions intended to manage gut health focus on the avoidance of antinutrients, toxins and excess protein, and the provision of anti-microbial or immune system-stimulatory products. Surprisingly, there is only limited focus on the role that fibre plays in maintaining gut health in broilers (and far more interest in the antinutritive role it can play) and yet it is probably the single most important dietary determinant of intestinal health. One reason for this situation is that the understanding of fibre is poor, made worse by the fact that the routine methods we use for determination of fibre have little value in describing the biological effects it conveys. As such, the fibre content and quality of a diet does not have the same level of scrutiny that other nutrients enjoy, e.g. amino acids. A far better understanding of the dietary content of the different components of fibre and an allocation of biological effect to each is essential if nutritionists are to take full advantage of its value in AGP-free diets. This paper focuses on why fibre is so important, what types of fibre we should consider, and how to ensure that the bird starts well and “learns” how to adapt its microbiota over time and as quickly as possible to utilise fibre as a health promoting substrate.
Fibre chemistry
Ideally, the nutritionist should be aware of the fractions of fibre that are rapidly, moderately, and slowly fermented and those fractions which have direct positive (e.g. gizzard development) or negative (e.g. viscosity, accelerating feed transit) effect on the digestive process. Unfortunately, even when the feed industry does consider fibre, it tends to focus on measurements based on methods developed over 150 years ago. This identifies dietary fibre as either crude fibre or nitrogen free extract. Neither of these categories convey any indication of structure or functionality. More sophisticated methods developed in the latter half of last century split fibre components into those fractions remaining after a wash with acid or neutral detergents. This does yield more useful information regarding the quantities of “intransigent” and potentially fermentable fibre components but this information is still quite rudimentary. More recent methods to separate the fibre into:
- Oligosaccharides
- Pectins
- Hemicellulose
- Cellulose
- Lignin.
This further categorization of fibre is somewhat helpful in identifying whether it might be fermented rapidly, slowly, or not at all, but information is still lacking. Further fundamental characteristics of fibre which influence the effects that it will exert in the intestinal tract of the monogastric are still overlooked. The solubility and size of the fibre along with its complexity of structure have an enormous influence on whether, and if so, where in the intestinal tract, it will be fermented. Each of these characteristics are discussed in more detail below.
Fibre solubility and size
Recent work has demonstrated that only soluble material and extremely small particulates get into the caecum of the chicken (Vanderghinste et al., 2024). The particulates on average are less than 50 microns which limits entry to a very small fraction of the insoluble ileal indigesta. It also suggests that technological processing of the diet likely has little influence on caecal entrance of insoluble fibrous materials. Indeed, to all intents and purposes the caeca should be seen as an organ which mostly processes soluble fibre, thus processes or additives which alter fibre solubility are potential tools for optimising large intestinal health in poultry.
Coupled with the solubility of the fibre is its molecular size. Very small molecules, oligosaccharides, are far more fermentable than their larger counterparts as the latter have to be disassembled before they can be absorbed into the bacterial cell and metabolised (Figure 1). The larger the molecule, the longer the time needed for disassembly. The initial rate of fermentation is therefore proportionately linked to the size of the molecule but there are two further considerations.

The first is that some oligosaccharides have recently been shown to act as signalling molecules in addition to them being readily fermentable. These “stimbiotics”, such as xylo- and arabinoxylo-oligosaccharides (XOS, AXOS) have been shown to dramatically influence the metabolic activity of many significant bacterial species involved in the deconstruction, transport and metabolism of fibre. (Amir et al., 2023). As such they not only increase the capacity of the caeca to degrade and effectively “digest” fibre on behalf of the host, but they also accelerate the development of such a microbiota structure so that the ability to degrade fibre is established earlier in the life of the host. Thus, the evolution of the large intestinal microbiota from a predominantly starch and protein degrading structure to a more stable fibre degrading structure is enhanced and stabilised. This concept is discussed in more detail below.
The second consideration is that very large and soluble polysaccharides, in addition to being sources of more slowly fermented fibre (which is desirable as noted in the next section), they may be detrimental due to their ability (in some cases) to aggregate and form viscous complexes. This can reduce digestibility of all nutrients dramatically and with regards to caecal fermentation and health, the key issue here is whether protein digestion is compromised to the point that the caeca is exposed to excessive soluble protein ingress. Putrefaction of this material can significantly degrade fibre fermentation and result in loss of intestinal integrity and even disease outbreak.
Complexity of fibre structure
The structural complexity of fibre influences the rate at which it can be depolymerised and subsequently metabolised. In general, linear NSP’s with very few substitutions can be depolymerised rapidly by just a single, relevant endo-acting enzyme. However, the presence of a few substitutions can limit access of the endo-enzymes to the backbone and thus reduce the rate and extent of depolymerisation. As substitution density and complexity increases, the rate of depolymerisation decreases and the size of the fragments produced increases. Highly substituted fibre demands a significant array of ancillary enzymes that need removal before the backbone can be attacked and the desired oligomers produced. The production of this wide array of ancillary enzymes requires the development of a complex and co-ordinated microbiota which takes time to develop. In general, the most complex fibre structures are fermented only once the “easy to ferment” material has been exhausted. A schematic of fibre complexity is shown in Figure 2.
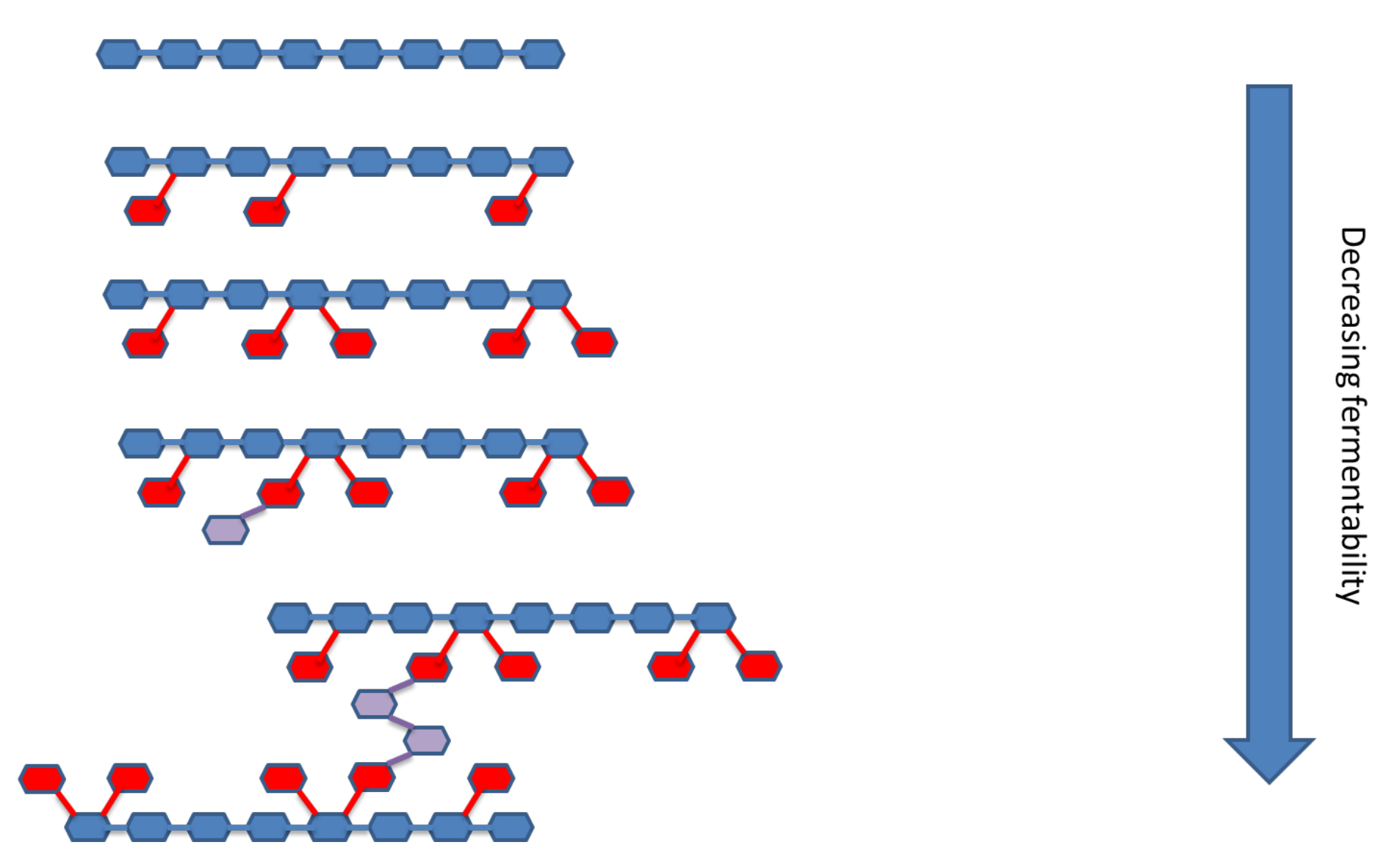
Evolution of the host microbiota
At hatch the intestines of the bird are sterile. In modern poultry production the newly hatched chick gets its initial inocula at the hatchery, during transport to the growing sheds and from the sheds themselves including the feeders (and feed) and waterers and numerous other sources. As a result, the structure of the intestinal microbiota is far more variant between individuals and flocks and less suitable for the development of a healthy microbiota compared with nature, where the hens caecal droppings around the nest provide an inocula of relevance to the environment in which the bird has grown. The development of a stable and beneficial microbiota is therefore not assured, and given the absence of prophylactic antibiotic use in many geographies, the opportunities for pathogens to establish are significant. Thus, the strategy for the industry is to ensure a smooth and rapid transition from initial colonizing bacteria to a healthy fibre fermenting community as rapidly as possible.

The evolution of the caecal microbiota is dependent not only upon the initial inocula, but also the substrates to which the bacteria are exposed. The material entering the caeca are the soluble indigesta from the ileum and given the digestive process in the neonate is not fully developed, this means that the caecal residents have a range of substrates to “chose” from, including starch and protein in relative abundance. The availability of such rapidly fermentable substrates favours the development of species which can utilise such material at the expense of those that specialise on more intransigent fibre sources. However, with time the chick significantly increases its ability to digest starch and protein, thus progressively limiting their delivery to the caeca. Coupled with the development of the host digestive capacity, there is also a development of the small intestinal microbiota which effectively remove not only starch and protein but also the more readily fermentable NSP (Davies et al., 2024). At the same time they release soluble NSP from the cell wall structures (Lee et al., 2017) and provide the caeca with more substrate, some of which is highly fermentable (Bai et al., 2024). As the ileal populations mature the supply of soluble fibre to the caeca stabilises with the majority being represented by arabinoxylan, galactan and mannan. Comparison of the sugar composition of ileal soluble fibre with that of the caeca suggests the arabinoxylan is by far the preferred substrate at 42 d of age (Lee et al., 2017) and hence the goal should be to develop arabinoxylan fermentation capability as soon as possible. It is most important to recognise that an ideal feed should contain rapidly, moderately and slowly fermented fibre so that all regions of the intestine are presented with substrate. As the bird ages and the caeca mature the availability of greater quantities of slowly fermented fibre may be critical for continued caecal health.
Priming of the caecal microbiota
From the points made above, it is clear that the sooner the caecal microbiota are adapted to ferment arabinoxylans, the smoother the transition from the neonatal microbiome to a stable adult microbiome. It has been known for a while that exposure of a broiler to a xylanase from first day of age markedly increases the ability of the caecal microbiome to utilise xylose, XOS, soluble arabinoxylans and even insoluble arabinoxylans (Bedford and Apajalahti, 2018). This clearly suggests that priming of the caecal microbiota is indeed possible, but it is not clear what it is that initiates the change. Prebiotic supply (through degradation and thus dissolution of insoluble NSP coupled with depolymerisation of high molecular weight soluble NSP) has been proposed as one of the 3 key mechanisms by which NSPase’s function in addition to viscosity reduction and cell wall degradation. Such supply of additional soluble NSP alone would presumably encourage the growth and activity of NSP degrading bacteria in the caeca, which could explain such effects. Indeed NSPases, particularly xylanases, should be of benefit in diets which are limited in soluble fibre content (eg Maize-soy and in particular sorghum-soy diets). However, whether simply supplying additional substrate is sufficient to drive a smooth caecal population transition before the alternative and rapidly disappearing substrates (protein and starch, e.g.) are exhausted, is not clear. Addition of 0.25% AXOS was shown to enrich Bifidobacteria and improve growth rates of broilers fed wheat-based diet suggesting that quantitatively supplying such a substrate may be all that is needed (Courtin et al., 2008). Indeed, addition of 0.5% AXOS to a wheat-based diet resulted in a significant acceleration of NSP fermentation in broilers compared with the control, suggesting not only was the AXOS fermented but also some of the structural NSP in the diet (Bautil et al., 2020). The AXOS was termed a “kickstarter” as it enabled NSP hydrolysis in the ileum and fermentation in the caecum at far younger ages than could be achieved on the control diet. However, concurrent work had shown such effects were possible with much lower addition rates of XOS – 60 g per tonne of feed. This is far too low an inclusion rate to result in a meaningful increment in fermentation activity directly (Ribeiro et al., 2018). Indeed, in this work it was suggested added XOS or AXOS generated by exogenous enzymes may alter the metabolism of the resident microbiota. Recent work has corroborated this hypothesis where it was shown that supply of as little as 50 g per tonne of feed of a XOS (DP2-6) markedly increased the presence of SusC and SusE proteins involved in the breakdown of complex polysaccharides, binding of the oligosaccharides produced at the outer membrane and transport across the periplasmic space (Amir et al., 2023). Thus it now appears that some of the products of NSPases may not only be prebiotic substrates, but also signalling molecules or “Stimbiotics” (Gonzalez-Ortiz et al., 2019), which radically alter the ability of the caecal microbiota to ferment fibre when present at very low levels in the diet. Whilst these molecules can be produced by xylanases in wheat based diets, they are not produced in corn based diets (Kouzounis et al., 2021), and hence for the stimbiotic effect in corn based diets, external XOS may have to be added. It is likely that differences in xylan substitutions limit the ability of xylanases to break AX down to the relevant DP2-6 XOS hence stimbiotic oligosaccharides are not produced. Indeed not all wheat samples release XOS when treated with a xylanase (Whiting et al., 2023) and hence XOS should be added as an insurance if the stimbiotic effect is to be guaranteed.
Priming – The challenge and problems for the industry
As the bird and the microbiota mature, the number of different carbohydrate sources entering the caeca become reduced such that at maturity the majority of the fermentable carbohydrate is xylan (Lee et al., 2017). If the caecal resident microbiota fail to adapt in time to use the AX then protein becomes the fermentation substrate of choice (Apajalahti and Vienola, 2016) which results in production of destructive metabolites and increases the chance of disease outbreak. The challenge is to ensure a rapid and smooth transition towards a microbiota capable of utilising the major fibre substrate in the caeca, namely AX, which can be achieved by educating or kickstarting their metabolism as soon as possible by provision of the necessary signalling molecules, namely the AXOS or XOS with low degrees of polymerisation. Once activated, the challenge for the industry is to maintain an adequate flow of soluble AX into the caeca, since insoluble material will likely not be able to enter due to the significant sieving effects of the caecal villi at the caecal entrance (Vanderghinste et al., 2024). It is proposed that a standard corn or sorghum soy diet may be lacking in this regard due to the paucity of soluble AX in each of these grains and as such the utilisation of a xylanase capable of degrading insoluble AX into soluble AX, particularly in the grower and finisher diets where fermentation capacity is maximised, is likely of great benefit. Thus a combination of a XOS or AXOS with a xylanase likely seems to be the most robust strategy for maintaining caecal health particularly in corn or sorghum based diets and perhaps even in wheat based diets where generation of XOS from a xylanase cannot be guaranteed (Whiting et al., 2023).
Conclusion
Nutritionists need to consider the evolution of the needs of the caecal microbiota in their diet formulations more than ever now that the “cure all”, i.e. prophylactic antibiotics, are no longer available in many parts of the world to deal the upsets that occur when fermentation balance is lost. A long-term approach will likely be most successful whereby the nutritionist not only matches the nutrient contents of the diet to suit the requirements of the bird during each phase, but also considers the quantity and type of fibre that should be present in order to encourage the establishment and development of a beneficial caecal microbiota.
References are available on request
From the Proceedings of the Australian Poultry Science Symposium 2025, by courtesy of the Poultry Research Foundation

















