The European Centre for Disease Prevention and Control (ECDC) has released a new scenario-based framework to support EU and EEA countries in preparing for, detecting, and responding to zoonotic influenza events with pandemic potential. The guidance, published on 4 December 2025, follows reports by the European Food Safety Authority (EFSA) of unprecedented levels of HPAI A(H5N1) circulation in wild birds during the 2025 autumn migration, which highlighted the need for strengthened preparedness and coordinated public health action.
The document, Scenarios for pre-pandemic zoonotic influenza preparedness and response in the EU/EEA, provides a structured approach to help authorities translate early signals of zoonotic influenza activity into proportionate and timely public health measures. The framework is intended for national public health authorities, preparedness planners, risk assessors and managers, policy-makers, laboratories, and clinical stakeholders across the EU/EEA.
A scenario system built on epidemiological and virological factors
The framework defines 14 scenarios, each based on combinations of key epidemiological and virological parameters, including:
-
animal origin (birds or mammals),
-
the number and exposure context of human cases,
-
severity indicators,
-
the presence of genetic markers associated with mammalian adaptation,
-
antiviral resistance, and
-
mismatch with available pre-pandemic vaccines or candidate vaccine viruses.
Each scenario is scored using a composite system (0–15) reflecting potential for onward human-to-human transmission, virological adaptation, and severity. These scores help guide the level of public health action required. A diagram summarising the scoring outcomes appears on page 14 of the guide.
A downloadable Excel tool, referenced in the document, allows countries to define and score scenarios using early triggers.
Baseline, escalating, and heightened alert actions
The guidance distinguishes three types of public health actions:
-
Baseline actions, which should be in place under the current epidemiological situation.
-
Escalating actions, activated when human cases occur in the EU/EEA or when certain virological or epidemiological indicators emerge.
-
Heightened alert actions, relevant when concerning scenarios occur outside the EU/EEA and may pose a risk of importation.
Actions span several domains:
-
preparedness and public health interventions,
-
surveillance and outbreak investigation,
-
laboratory preparedness and functions,
-
One Health coordination,
-
occupational safety,
-
risk communication,
-
use of antivirals,
-
vaccination, and
-
additional studies.
Annexes 2 and 3 of the guide present comprehensive tables listing baseline and escalating actions for each domain.
Integration with One Health investigations
The framework complements the previously published ECDC/EFSA One Health guidance for zoonotic avian influenza. While the One Health document outlines how cross-sectoral investigations should be initiated and organised, the new scenario framework specifies which public health measures should escalate depending on the outputs of these investigations. This relationship is illustrated in the diagram on page 11.
The guidance underscores the importance of:
-
cross-sectoral communication between public health and veterinary authorities,
-
rapid sharing of virus sequences with international databases,
-
and coordination with EFSA, EMA, EU-OSHA, WHO, FAO and WOAH.
Surveillance, laboratory readiness, and occupational safety
The document places strong emphasis on surveillance system strengthening, including:
-
event-based surveillance,
-
monitoring of exposed occupational groups,
-
wastewater and environmental surveillance,
-
and timely outbreak investigations.
In the laboratory sector, the framework details baseline and escalating actions for both national reference laboratories and diagnostic/hospital laboratories. These include assay validation, biosafety requirements, specimen referral pathways, capacity-building, and readiness to scale up diagnostic and sequencing capacity.
The framework also outlines occupational safety measures in line with Directive 2000/54/EC, including worker risk assessments, PPE requirements, hygiene measures, and procedures for managing contaminated environments in agricultural and high-risk settings.
Antiviral and vaccination considerations
The guidance describes the use of antivirals for treatment and post-exposure prophylaxis (PEP), with escalation depending on scenario characteristics such as severity signals or detection of mammalian adaptation.
Vaccination strategies include:
-
continuation of seasonal influenza vaccination,
-
optional use of zoonotic influenza vaccines for at-risk groups at baseline,
-
potential expansion of A(H5) vaccine use under escalating scenarios,
-
and alignment with the provisional WHO SAGE recommendations on A(H5) vaccination from September 2025.
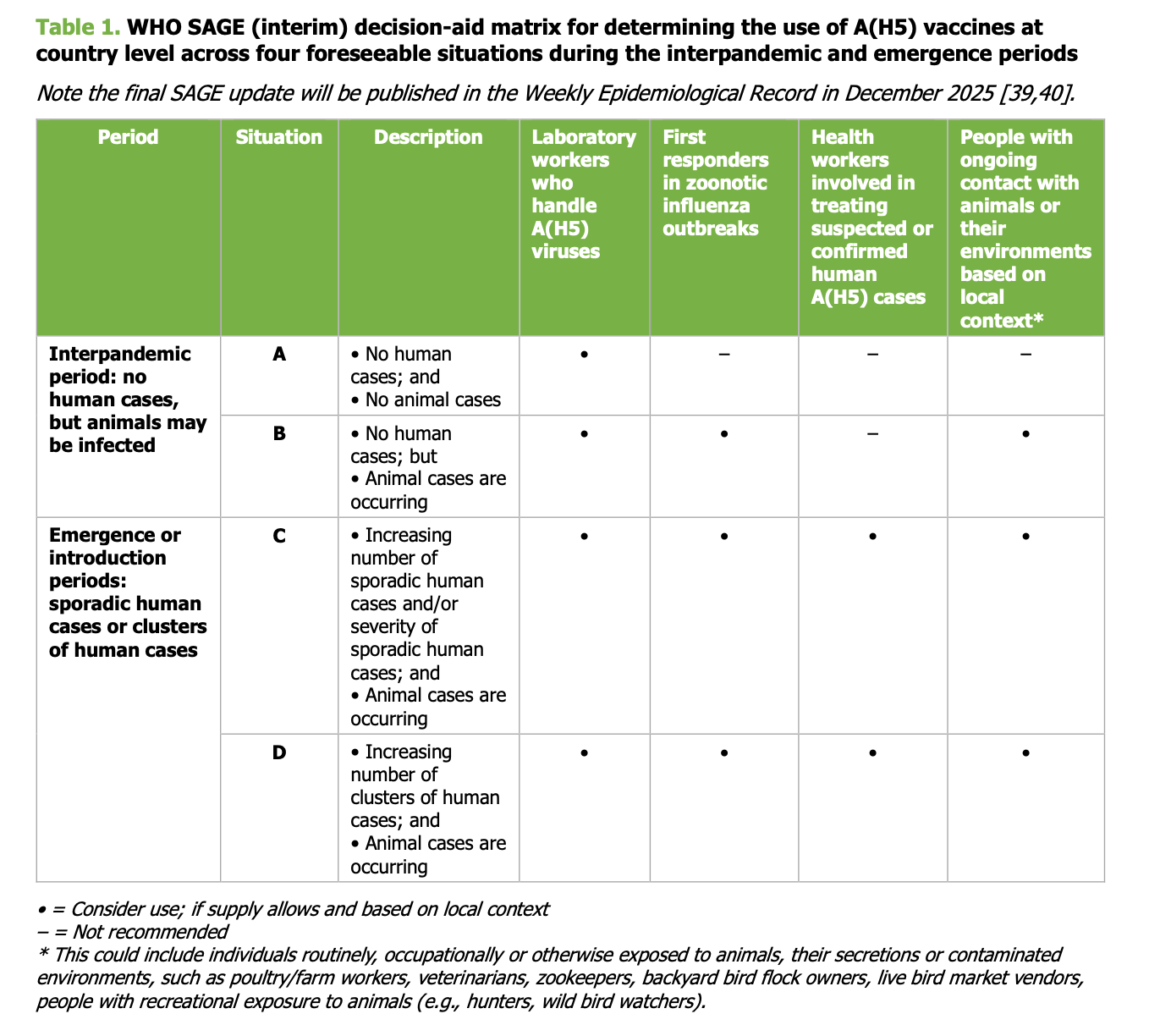
The WHO decision-aid matrix for A(H5) vaccine use appears in Table 1 on page 21.
 A shared framework to strengthen EU/EEA preparedness
A shared framework to strengthen EU/EEA preparedness
The ECDC notes that the scenario-based framework does not replace formal pandemic risk assessment tools (e.g., WHO TIPRA, US CDC IRAT) and is not designed to predict pandemics. Instead, it supports early-stage situational awareness, operational planning, and coordinated decision-making when zoonotic influenza signals emerge.
By presenting common scenarios and associated public health actions, the framework enables EU/EEA countries to identify preparedness gaps, improve coordination, and align response measures as the epidemiological situation evolves.
Source: www.ecdc.europa.eu