Coccidiosis is a complex disease in chickens caused by protozoan parasites in the genus Eimeria. As Eimeria species are ubiquitous in poultry facilities, coccidiosis is one of the most common and prevalent diseases in commercial poultry operation. Conducting health surveys, posting sessions, and OPG counts on a regular basis plays a crucial role in understanding the gut health of the flock.
➤ Vijay Durairaj1, Nicholas Brown2, Daniel Coleman1, Emily Barber1, and Ryan Vander Veen1
1Huvepharma, Inc., Lincoln, Nebraska, USA
2Huvepharma, Inc., Peachtree City, Georgia, USA
Corresponding author: Vijay.Durairaj@huvepharma.us
The profitability of the poultry industry depends on the overall flock health and production performance. Coccidiosis adversely affects intestinal health contributing to morbidities, mortalities, and prophylactic/therapeutic costs. With a significant economic impact, coccidiosis is one of the biggest challenges faced by the poultry industry. This case report details clinical coccidiosis in broilers associated with E. maxima and E. praecox progressing to necrotic enteritis.
Introduction
Coccidiosis is one of the most common intestinal diseases of chickens caused by protozoan parasites of genus Eimeria. Among the nine Eimeria species described, the seven recognized globally are E. acervulina, E. brunetti, E. maxima, E. mitis, E. necatrix, E. praecox, and E. tenella (5). Coccidiosis causes malabsorption, enteritis, impaired feed utilization, poor feed conversion ratio (FCR), reduced weight gain, and mortalities. It also increases susceptibility to secondary bacterial infections. Coccidiosis poses a burdensome challenge to the modern poultry production system by affecting short as well as long-lived birds. Coccidiosis inflicts substantial economic losses on the poultry industry. The economic impact of coccidiosis is not limited to the lowered production performances and mortalities, but also includes the cost of prophylactic and therapeutic measures.
In a 2022 USA survey, coccidiosis ranked number one in broilers and cage free layers, while it ranked number two in caged layers (11). Coccidiosis is one of the top concerns of antibiotic free (ABF), raised without antibiotics (RWA), and no antibiotics ever (NAE) commercial poultry operations.
Eimeria species are ubiquitous in commercial poultry operations. Poor litter management, high humidity in the barn, and high stocking density are considered as risk factors contributing to subclinical/clinical coccidiosis. Higher oocyst burden in the litter and shorter downtime periods are potential risk factors for coccidiosis. Husbandry, management, biosecurity and sanitization practices, in conjunction with anticoccidial prophylactic/ therapeutic measures, help to keep coccidiosis under control.
“Posting sessions” are routinely performed in commercial poultry operations and “cocci check” is one way to assess the intestinal health. “Cocci check” helps in detecting the intestinal lesions and is a valuable tool to assess anticoccidial treatment/coccidiosis vaccine program. Oocysts per gram (OPG) helps in understanding the shedding of Eimeria oocysts in the barn. Diagnostic evaluation of oocysts helps in identifying circulating Eimeria strains in the field. Proactive measures to combat coccidiosis can be implemented based on these findings. In some cases, subclinical or clinical coccidiosis can be seen as early as the second week after placement depending on the Eimeria oocyst burden in the litter. This case report details a field investigation study of clinical coccidiosis in broilers.
Case report
Case history and field investigation
In Spring 2023, a broiler complex with two houses in Southeast USA had enteric issues starting from second week of age. At 20 days of age, a field investigation was conducted. A cumulative mortality of 1193/60900 birds (1.96%) was documented in house 1. Birds with clinical signs were euthanized and necropsied. Postmortem investigation of dead birds was also conducted on site.
Evaluation of mucosal scrapings
The intestinal mucosal scrapings were collected from the necropsied birds and visualized under a microscope.
Sporulation of oocysts
The intestinal contents from the necropsied birds were homogenized and mixed with 2.5% potassium dichromate and sporulated in aerated flasks placed in a shaking incubator at 28°C for 48 hours (12).
DNA extraction, PCR, and gel electrophoresis
DNA was extracted from the oocysts by using glass beads to rupture them followed by proteinase K (Qiagen) digestion. The DNA extraction was performed by using QIAamp Fast DNA Stool Kit (Qiagen) following manufacturer recommendations. Each PCR reaction (25 µL) had 5 µL of the template along with 1x GoTaq® G2 Hot Start Master Mix (Promega) and 0.4 µM of each primer. The amplicons (2 µl) generated from the PCR reactions were electrophoresed and visualized in E-Gel™ EX Agarose Gels, 2% (Invitrogen). PCR was performed against E. brunetti, E. necatrix, and E. tenella (6), E. praecox, E. mitis (10), E. maxima and E. acervulina using in-house primers. A reference E-Gel™ 1 Kb Plus DNA Ladder (Invitrogen) was used to identify the size of the amplicons. The amplicons were purified following the instructions using a QIAquick PCR purification kit (Qiagen) followed by submission for sequencing (Eurofins, KY).
Results and discussion
On field investigation, a few sick birds were euthanized and necropsied. Necropsied birds had ballooned intestines with flaccid tone (Figure 1.A). The serosa had numerous pinpoint petechiae (Figure 1.B) and the intestinal lumen had orange mucoid contents (Figure 1.C).
 In the necropsied birds, the intestinal lesions were very severe with roughened mucosa and velvety (“turkish towel”) appearance suggestive of necrotic enteritis (Figure 2).
In the necropsied birds, the intestinal lesions were very severe with roughened mucosa and velvety (“turkish towel”) appearance suggestive of necrotic enteritis (Figure 2).
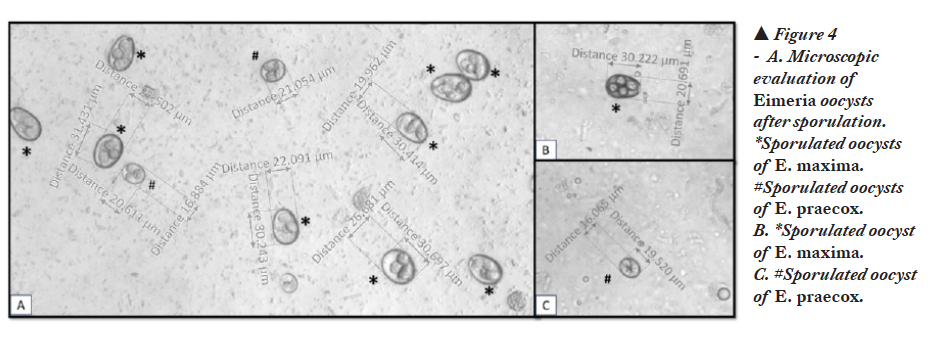
On lab investigation, oocysts of E. maxima and E. praecox were identified in mucosal scrapings (Figure 3.A, B, C) and sporulated (Figure 4.A, B, C).
The oocysts of E. maxima are ovoid shape and larger in size (30.5×20.7 µm) compared to other Eimeria species of chickens. The oocysts of E. maxima are easy to identify due to their larger size. The oocysts of E. praecox are ovoidal shape with an average size of 21.3×17.1 µm. Both species of Eimeria were confirmed by PCR (Figure 5.A, B, C).
 Based on the severity of the disease, coccidiosis can be classified into three types: coccidiasis, subclinical coccidiosis and clinical coccidiosis (14). Coccidiasis is a mild type of infection without causing any adverse effects (9). Subclinical coccidiosis circulates in the farm without clinical signs or unnoticeable very mild clinical signs and has a moderate impact on the production parameters and FCR. The subclinical form is the most prevalent form of coccidiosis and remains a subtle challenge to the poultry industry. It is very difficult to diagnose subclinical coccidiosis. Clinical coccidiosis exhibits clinical signs such as watery or bloody droppings, soiled vent, depression, and huddling. It drastically affects the production parameters, growth rate, FCR, and increases mortalities.
Based on the severity of the disease, coccidiosis can be classified into three types: coccidiasis, subclinical coccidiosis and clinical coccidiosis (14). Coccidiasis is a mild type of infection without causing any adverse effects (9). Subclinical coccidiosis circulates in the farm without clinical signs or unnoticeable very mild clinical signs and has a moderate impact on the production parameters and FCR. The subclinical form is the most prevalent form of coccidiosis and remains a subtle challenge to the poultry industry. It is very difficult to diagnose subclinical coccidiosis. Clinical coccidiosis exhibits clinical signs such as watery or bloody droppings, soiled vent, depression, and huddling. It drastically affects the production parameters, growth rate, FCR, and increases mortalities.
E. maxima is one of the most common Eimeria species documented in chickens (4, 13). E. maxima is classified as a moderate to highly pathogenic Eimeria species, inducing moderate to severe lesions (5, 14). It affects the mid-small intestine and induces lesions in jejunum and ileum. In severe infections, the lesions may extend throughout the small intestine. Serosal surface may have a few small red petechiae to numerous petechiae. Intestinal lumen may be filled with yellow-orange mucus to bloody contents. The mucosal surface may be roughened and have mucus and blood clots. The pathological manifestations of E. maxima can induce ballooning of intestine. In severe infections, the intestine may be loaded with bloody contents (5, 8). E. maxima infection results in poor feed conversion, poor weight gain, diarrhea, and mortalities.
E. praecox is classified as a less pathogenic species, inducing no lesions or very minimal lesions (2, 14). E. praecox affects duodenum and does not induce any notable lesions in the intestine (5). Heavy infection of E. praecox may result in pinpoint hemorrhage in the mucosa of the duodenum and intestinal contents may be watery. Heavy infection of E. praecox may result in reduced weight gain and poor feed conversation (2, 5).
Eimeria replicates in the intestine and damages the intestinal epithelium, compromising intestinal function as well as integrity. This provides an opportunity and niche for secondary bacterial pathogens to invade and colonize the intestine. Pathogenic bacteria such as Clostridium perfringens type A can use this opportunity resulting in necrotic enteritis (3,14). Coccidiosis followed by secondary bacterial infections influences the microbiome diversity and can result in conditions such as dysbacteriosis and necrotic enteritis. Concurrent Eimeria infections are common in poultry operations (1,7) as well as concurrent Eimeria infections and secondary bacterial infections (3,14). In this clinical case, severe infection of E. maxima resulted in necrotic enteritis. A concurrent infection of E. praecox was also documented in this case. Both coccidiosis and necrotic enteritis cause significant economic losses to the poultry industry.
Several intervention strategies such as synthetic compounds/chemicals, ionophores, natural alternative products, and vaccines are used to mitigate coccidiosis in commercial poultry operations. In modern poultry production facilities, conducting “health surveys,” “posting sessions” and “OPG counts” on a regular basis plays a crucial role in understanding the gut health of the flock and initiating proactive measures to prevent intestinal diseases, especially coccidiosis.
Acknowledgements
We thank the poultry producer for providing the opportunity to study the coccidiosis outbreak in this flock. We extend thanks to Trish Gray for her support.
Click here to download the PDF version of this article.
References
- Aarthi S, Raj GD, Raman M, Blake D, Subramaniam C, Tomley F. Expressed sequence tags from Eimeria brunetti–preliminary analysis and functional annotation. Parasitol Res. 2011 Apr;108(4):1059-62.
- Allen PC, Jenkins MC. Observations on the gross pathology of Eimeria praecox infections in chickens. Avian Dis. 2010 Jun;54(2):834-40.
- Al-Sheikhly F, Al-Saieg A. Role of Coccidia in the occurrence of necrotic enteritis of chickens. Avian Dis. 1980 Apr-Jun;24(2):324-33.
- Blake DP, Marugan-Hernandez V, Tomley FM. Spotlight on avian pathology: Eimeria and the disease coccidiosis. Avian Pathol. 2021 Apr 20:1-5.
- Cervantes HM, McDougald, LR, Jenkins MC. Coccidiosis. In: Swayne D, Boulianne, M, Logue C, McDougald L, Nair V, Suarez D, deWit S, Grimes T, Johnson D, Kromm M, et al., editors. Diseases of poultry. 14th ed. Ames (IA): Wiley-Blackwell. 2020. 1193–1216.
- Gautam Patra, M. Ayub Ali, Kh. Victoria Chanu, L. Jonathan, L. K. Joy, M. Prava, R. Ravindran, G. Das and L. Inaotombi Devi. PCR based diagnosis of Eimeria tenella infection in broiler chicken. International Journal of Poultry Science. 2010. 9: 813-818.
- Györke A, Pop L, Cozma V. Prevalence and distribution of Eimeria species in broiler chicken farms of different capacities. Parasite. 2013. 20:50. doi: 10.1051/parasite/2013052.
- Johnson J, Reid WM. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol. 1970. Aug;28(1):30-6.
- Levine, N.D. Protozoan Parasites of Domestic Animals and Man. Minneapolis: Burgess Publishing Company.1961.
- Schnitzler BE, Thebo PL, Tomley FM, Uggla A, Shirley MW. PCR identification of chicken Eimeria: a simplified read-out. Avian Pathol. 1999. Feb;28(1):89-93.
- USAHA. Report of the USAHA committee on poultry and other avian species. United States Animal Health Association. 2022. https://usaha.org/transmissible-diseases-of-poultry-avian-species/.
- Venkateswara Rao P, Raman M, Gomathinayagam S. Sporulation dynamics of poultry Eimeria oocysts in Chennai. J Parasit Dis. 2015. Dec;39(4):689-92.
- Wang M, Tian D, Xu L, Lu M, Yan R, Li X, Song X Protective efficacy induced by Eimeria maxima rhomboid-like protein 1 against homologous infection. Front Vet Sci. 2023. Jan 4;9:1049551.
- Williams RB. Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 2005. Jun;34(3):159-80.