Blackhead disease (histomoniasis) in turkeys is one of the protozoan diseases sporadically documented in commercial turkey production facilities. Histomoniasis may induce mild, moderate, or severe mortalities up to 80-100%. With the absence of commercial vaccines and prophylactic/therapeutic drugs to combat histomoniasis, epidemiology studies provide critical insights into the pathogenesis of this disease. This clinical case report details concurrent infection of Histomonas meleagridis and Eimeria meleagridis in a turkey barn.
➤ Vijay Durairaj1, Steven Clark2, Emily Barber1 and Ryan Vander Veen1
1 Huvepharma, Inc., Lincoln, NE 68528, USA
2 Huvepharma, Inc., Peachtree City, GA 30269, USA
Corresponding author: Vijay.Durairaj@huvepharma.us
Blackhead disease (histomoniasis, histomonosis, enterohepatitis) in turkeys is caused by an anaerobic protozoan parasite, Histomonas meleagridis (1). Turkeys are highly susceptible to histomoniasis compared to chickens. Heterakis gallinarum, the common cecal worm of gallinaceous birds, acts as an intermediate host for H. meleagridis. Earthworms serve as a paratenic host for H. meleagridis. Vectors such as insects, flies, and fomites have been documented as carriers of Heterakis gallinarum eggs.
H. meleagridis affects the ceca and enters the hepatic portal circulation resulting in necrosis of liver. The mortalities are highly variable in histomoniasis field outbreaks. Mortalities may be mild, moderate, or even severe causing up 80-100% mortalities (2). Several factors are associated with increased mortalities, including virulence of the isolate, age of the birds, season, immune status of the birds, and concurrent infections (3, 4). Histomoniasis associated mortalities, production losses and carcass condemnations cause substantial losses to the turkey industry (5).
For decades, blackhead disease was managed by administration of antihistomonas drugs. Due to food safety concerns and several other reasons, prophylactic and therapeutic measures for histomoniasis were either banned or withdrawn from the global market (6). Currently, there are no commercial vaccines or efficacious prophylactic/therapeutic drugs to combat histomoniasis. The AAAP research priorities of 2022 has ranked investigative studies related to histomoniasis epidemiology and pathogenesis as a top five research priority in the turkey industry (7).
This case report details a field investigation of blackhead disease outbreak in a turkey flock. An in-depth investigation is recommended in field cases to clearly understand the disease. Sometimes the disease etiology may be masked by or combined with other factors exhibiting an exaggerated manifestation of the disease. The field investigation study is a valuable tool to understand complicating factors involved in this disease.
Case report
Case history
In Fall 2022, a blackhead disease outbreak was reported in two barns in a 9 week-old turkey flock (12,300 turkeys per barn) located in the Midwestern United States. Each house was placed with 12,300 poults and the quality of the poults was considered poor at the time of placement. Turkeys received anti-coccidial feed additive Monensin, a polyether ionophore product. A pest control program was implemented on the farm. Poults were directly placed in the house and no litter amendments were used. Clinical signs such as dullness, depression, yellow stained vent, and ruffled feathers were noticed during a farm investigation. Dead birds were necropsied and gross lesions were observed in the ceca and liver. A presumptive diagnosis of blackhead disease was made based on the gross lesions. Turkeys exhibiting clinical signs were euthanized, necropsied and samples were collected.
Culture
Small pieces of cecal samples were collected from the necropsied turkeys and transported in plug seal-capped T25 flasks with 10 mL modified Dwyer’s media (8) with 0.8% (w/v) rice powder, 0.35% (wt/vol) sodium bicarbonate (Sigma-Aldrich, St. Louis, MO), and 5% horse serum (HyClone, Logan, UT) in Medium 199 powder (Gibco, Grand Island, NY). The culture flasks were shipped in a warm insulated Styrofoam box. The culture flasks were incubated at 40 ˚C for 2 days.
Intestine samples
The intestine samples were collected in zip-lock bags and triple bagged along with ice packs. The intestine samples were shipped in a cold insulated box. The intestines were evaluated for gross lesions and mucosal scrapings were collected from the duodenum, ileum, and ceca and evaluated under the microscope.
DNA extraction and PCR
DNA was extracted from the ceca using the DNeasy® Blood & Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. Previously described primers were used to amplify partial 18S ribosomal RNA (rRNA) gene of H. meleagridis (9) and Heterakis gallinarum (10), and cytochrome c oxidase subunit I gene (COI) of Eimeria species using previously published primers (11) and in-house primers. All PCR reactions were performed using GoTaq® G2 Hot Start Green Master Mix (Promega, Madison, WI) and 5 µM of forward and reverse primers were used.
Gel electrophoresis and sequencing
After PCR amplification, the amplicons (2 µl) were electrophoresed and visualized on E-GelTM EX Agarose Gels, 2% (Invitrogen, Carlsbad, CA) using the E-Gel Power Snap Electrophoresis Device (Invitrogen). E-Gel 1 Kb Plus DNA Ladder (Invitrogen) was used as a reference to determine the size of the amplicons. The amplicons were purified using QIAquick PCR Purification Kit (Qiagen) and submitted for sequencing (Eurofins, Louisville, KY).
Results
Gross pathology
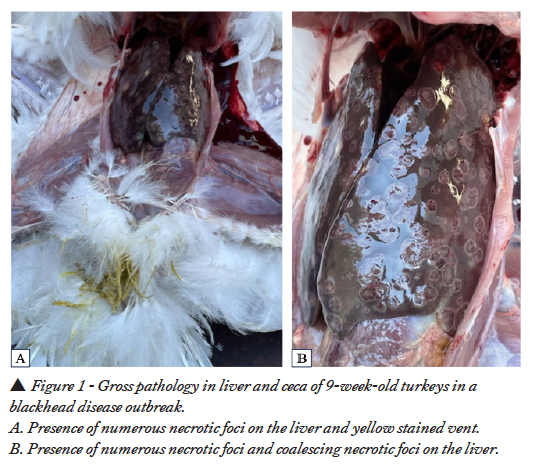
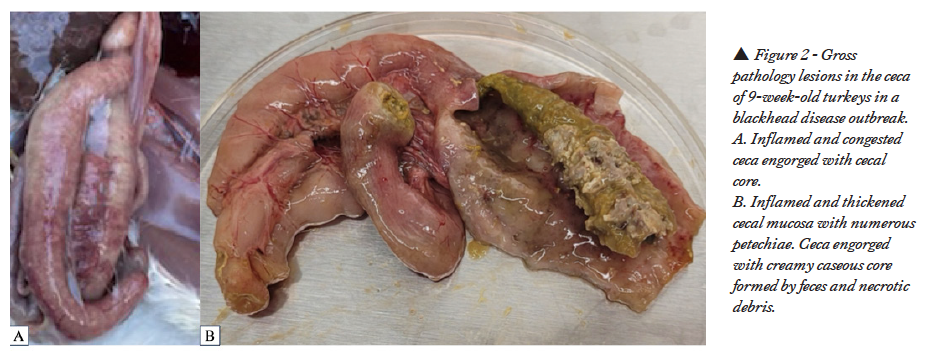
Necropsied turkeys had characteristic lesions in the liver and ceca suggesting histomoniasis. Multifocal to coalescent necrotic foci with dark centers and defined white periphery were noticed in the livers (Figures 1A, B). Cecal wall was thickened, hyperemic, and serosa inflamed with numerous petechiae and cecal core was visible without dissecting the ceca (Figure 2A). Cecal mucosa was thickened, edematous, necrotic and had numerous petechiae with creamy yellowish cecal core (Figure 2B).
Culture
The culture flasks were observed periodically under the inverted microscope. After two days of incubation at 40 ˚C under anaerobic conditions, H. meleagridis was observed in microscopic evaluation of the cecal culture (Figure 3A, B). H. meleagridis had amoeboid/ pulsative movements as described earlier (3,12). In addition to H. meleagridis, prolific growth of unidentified bacterial population was also detected in the flask.
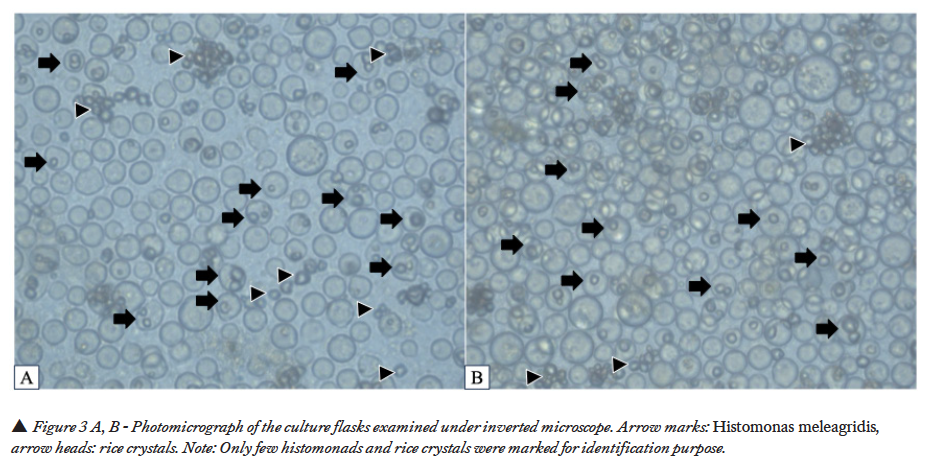 Intestinal samples
Intestinal samples
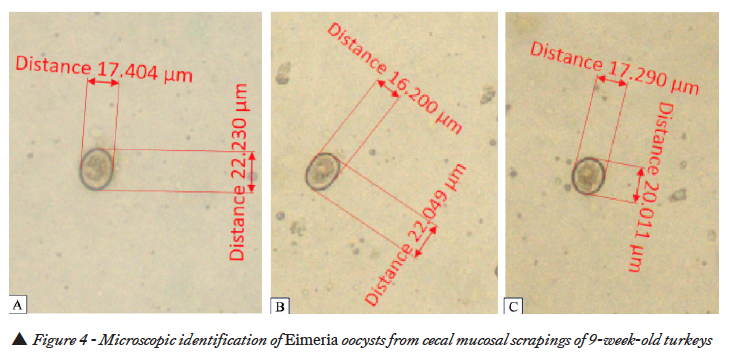
Intestinal samples received in cold Styrofoam boxes were examined and mucosal scrapings were collected from the ceca. Wet mount examination of the mucosal scrapings from the ceca revealed the presence of Eimeria oocysts (Figure 4A, B,C).
 PCR and gel electrophoresis
PCR and gel electrophoresis
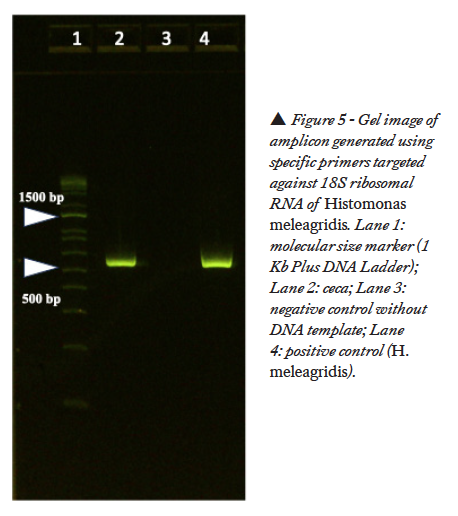
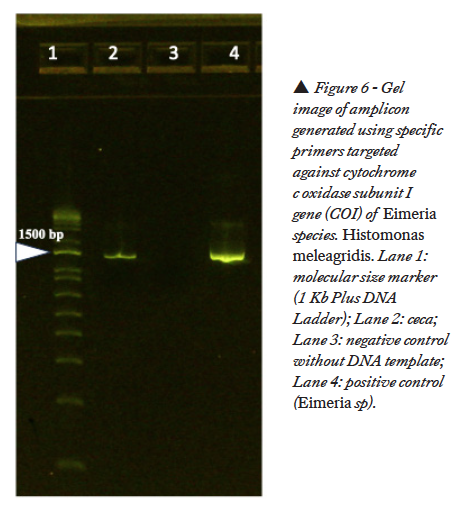
With the primers targeted against 18S ribosomal RNA of H. meleagridis, a positive band was noticed in the gel at 550 bp (Figure 5). No band was noticed in the gel with the primers targeted against H. gallinarum. With the primers targeted against cytochrome c oxidase subunit I gene (COI) of Eimeria species, a positive band was noticed at 1340 bp (Figure 6).
Sequencing
The purified gel products were submitted for sequencing. The sequences were analyzed using the National Center for Biotechnology Information database (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The gene targeted sequencing of the PCR product generated from PCR against 18S rRNA of H. meleagridis clustered with genotype 1 H. meleagridis isolates and was 95% identical to H. meleagridis isolate 11/6346 (GenBank accession no.: HG008087.1). The gene targeted sequencing of the PCR product generated from PCR against cytochrome c oxidase submit I gene (COI) of Eimeria species revealed that the field isolate was 99% identical to E. meleagridis (GenBank accession no.: KJ608418.1).
Discussion
Based on the gross lesions in the liver and ceca during onsite field investigation, a presumptive diagnosis of blackhead disease was made. PCR and culture confirmed H. meleagridis infection. In addition, evaluation of mucosal scrapings revealed the presence of Eimeria oocysts and confirmed as E. meleagridis by PCR.
In turkeys, bacterial diseases such as colibacillosis, avian pasteurellosis, avian mycobacteriosis, spotty liver disease, pullorum disease, fowl typhoid, and viral diseases such as inclusion body hepatitis, turkey viral hepatitis, avian leukosis, Marek’s disease, and parasitic diseases such as histomoniasis and tetratrichomoniasis, can induce lesions in the liver. Cecal lesions can be seen in bacterial diseases such as salmonellosis, and parasitic diseases such as tetratrichomoniasis and coccidiosis (E. adenoeides, E. gallopavonis, and E. meleagridis). Both liver and cecal lesions can be seen in salmonellosis, tetratrichomoniasis, and histomoniasis.
In the past, concurrent infection of histomoniasis has been reported in several cases. Concurrent infection with bacterial pathogens such as Escherichia coli (13), Salmonella typhimurium (13) and Brachyspira-like bacteria (14), viral pathogen such as Hemorrhagic enteritis virus (4), and parasitic pathogens such as Pentatrichomonas hominis (3) and Eimeria spp. (15) have been documented. In this case, concurrent infection of H. meleagridis and E. meleagridis was documented.
E. meleagridis was first described by Tyzzer in 1927 (16). The first generation schizonts of E. meleagridis occurs in the small intestine, while the other generations and gametogony occurs in the ceca (17,18). E. meleagridis is considered a nonpathogenic strain (19). However, studies conducted by Matsler and Chapman, concluded that E. meleagridis may cause reduction in weight gain and the pathogenicity classification of E. meleagridis should be reconsidered (18).
Regardless of the pathogenicity, the damage incurred in the cecal epithelium during E. meleagridis replication cannot be ignored. Compromised cecal epithelium provides an unchallenging entry site for H. meleagridis to invade the ceca (3,4). Synergistic and exaggerated damage may be noticed in H. meleagridis and concurrent infections with other pathogens. The interaction of H. meleagridis with other pathogens needs to be investigated in detail for a better understanding of the disease.
With the unavailability of commercial vaccines and prophylactic/therapeutic measures, understanding the interaction of H. meleagridis with other pathogens helps in implementing proactive measures to minimize the risk of complicating/aggravating factors.
References
- Cushman S. The production of turkeys. In: Bull. 25, Agricultural Experiment Station. Kingston: (RI): Rhode Island College of Agriculture and Mechanical Arts; p. 89–123; 1893.
- Hess M, McDougald LR. Histomoniasis. In: Swayne D, Boulianne M, Logue C, McDougald L, Nair V, Suarez D, deWit S, Grimes T, Johnson D, Kromm M, et al., editors. Diseases of poultry. 14th ed. Ames (IA): Wiley-Blackwell. p. 1223–1230; 2020.
- Durairaj V, Barber E, Clark S, Vander Veen R. Concurrent infection of Histomonas meleagridis and Pentatrichomonas hominis in a blackhead disease outbreak in turkeys. Avian Dis. 67:124–129; 2023.
- Durairaj V, Nezworski J, Drozd M, Clark S, Veen RV. Concurrent Histomonas meleagridis and Hemorrhagic Enteritis Virus Infection in a Turkey Flock with Recurrent History of Blackhead Disease. Avian Dis.56-64; 2024.
- Durairaj V, Carriere R, Drozd M, Lin G, Keyser K De, Vander Veen R. The impact of histomoniasis in organic turkeys. Poultry World. Vol 40. No. 2: 20-23. 2024.
- Clark, S., and L. Frobel. Current health and industry issues facing the US turkey industry. Proceedings of the 126th Annual Meeting of the United States Animal Health Association, Minneapolis, MN. p. 193–198; 2022.
- American Association of Avian Pathologists. 2022 Research Priorities of the American Association of Avian Pathologists. [modified 2022 Oct 10; accessed 2023 Mar 29]. https://aaap.memberclicks.net/assets/Committees/Research_Priorities/Research%20Priorities%202022.pdf; 2022.
- Hauck R, Armstrong PL, McDougald LR. Histomonas meleagridis (Protozoa: Trichomonadidae): analysis of growth requirements in vitro. J Parasitol. 96:1–7; 2010.
- Bilic I, Jaskulska B, Souillard R, Liebhart D, Hess M. Multi-locus typing of Histomonas meleagridis isolates demonstrates the existence of two different genotypes. PLOS ONE 9:e92438; 2014.
- Cupo KL, Beckstead RB. PCR detection of Heterakis gallinarum in environmental samples. Vet Parasitol. 271:1–6; 2019.
- Hafeez MA, Shivaramaiah S, Dorsey KM, Ogedengbe ME, El-Sherry S, Whale J, Cobean J, Barta JR. Simultaneous identification and DNA barcoding of six Eimeria species infecting turkeys using PCR primers targeting the mitochondrial cytochrome c oxidase subunit I (mtCOI) locus. Parasitol Res. 2015 May;114(5):1761-8.
- Tyzzer EE. The flagellate character and reclassification of the parasite producing ‘‘blackhead’’ in turkeys: Histomonas (gen. nov.) meleagridis (Smith). J Parasitol. 6:124–131; 1920.
- Ganapathy K, Salamat MH, Lee CC, Johara MY. Concurrent occurrence of salmonellosis, colibaccillosis and histomoniasis in a broiler flock fed with antibiotic-free commercial feed. Avian Pathol. 29:639–642; 2000.
- Esquenet C, De Herdt P, De Bosschere H, Ronsmans S, Ducatelle R, Van Erum J. An outbreak of histomoniasis in free-range layer hens. Avian Pathol. 32:305–308; 2003.
- Chadwick E, Beckstead R. Two blackhead disease outbreaks in commercial turkey flocks were potentially exacerbated by poor poult quality and coccidiosis. Avian Dis. 64:522–524; 2020.
- Tyzzer, E. E. Species and strains of coccidia in poultry. J. Parasitol. 13:215. 1927.
- Clarkson MJ. The life history and pathogenicity of Eimeria meleagridis Tyzzer, 1927, in the turkey poult. Parasitology. 1959 Nov;49:519-28.
- Matsler PL, Chapman HD. Characterization of a strain of Eimeria meleagridis from the turkey. Avian Dis. 2006 Dec;50(4):599-604.
- Lund, E.E, & Farr, M.M. (1965). Coccidiosis of the turkey. In H.E. Biester & L.H. Schwarte (Eds.), Diseases of Poultry, 5th ed., (pp. 1088-1093). Ames, IA: Iowa State University Press.