
The beneficial role of short-chain fatty acids and medium chain fatty acids on gut health and reducing pathogen colonization has been well documented over the past decade.
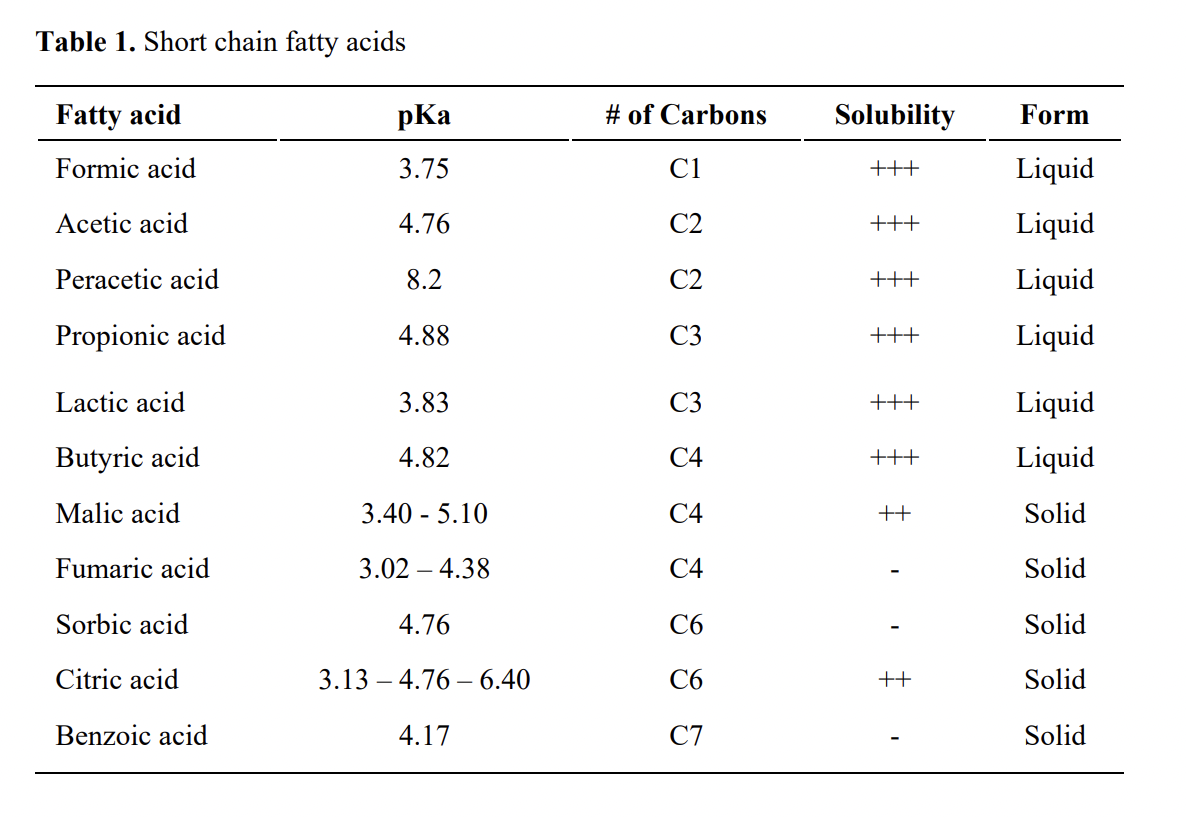
Short chain fatty acids (Table 1) are volatile fatty acids produced by the fermentative activity of the endogenous microflora in chickens including formic, acetic, propionic and butyric acids. In old research, SCFAs were attributed to inhibit the growth of Salmonella. Over past decade, formic acid, propionic, and butyric acid have been studied widely by various research groups. Propionic and butyric acids decrease the invasion of intestinal epithelial cells, whereas acetic acid and formic acid do not have this effect.
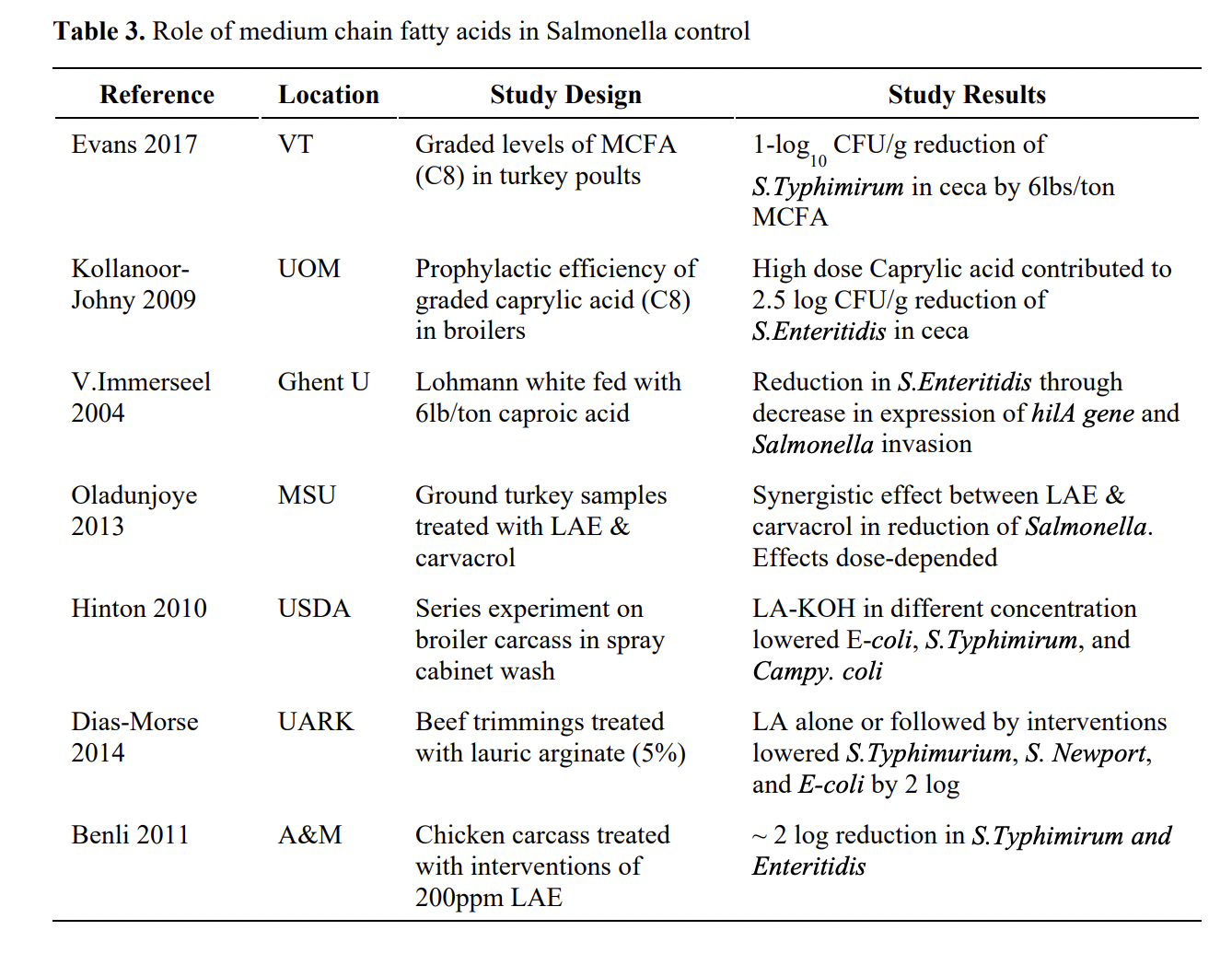
Since poultry feed could be a major source for Salmonella introduction to the farms, the direct addition of formic acid, propionic acid to feed and the resultant pathogenic load was investigated. Some of the published work exploring effect of MCFA on Salmonella control is summarized in Table 3.
Some literature suggested that, individual additions of these SCFAs did not significantly reduce the Salmonellashedding by birds, resulting in the commercial propagation of mixtures of propionic and formic acids. The antimicrobial properties of these mixtures were investigated in various parts of the gastrointestinal tract in chickens. In a study with broilers, Hinton and Linton (1988) observed that formic-propionic acid mixture at 0.5 and 0.68% w/w decreased S. Kedougou substantially between the control (22/27 positive) and treated (1/30 positive) groups. In another study, a significant 2.5 log reduction in cecal S. Typhimurium counts at 14-days of age, and >3.5 log reduction at 21-days of age were observed by the addition of 1% mix of propionate and formate in chicken diets. In addition, when chickens received a mixture of formic and propionic acids, significant reduction in the mortality of chickens with S. Pullorum and S. Gallinarum were observed.
On the contrary, in a study using formic/propionic acid blend (0.125 to 1%), Waldroup et al. (1995) reported that the mixture did not consistently reduce levels of nalidixic acid resistant Salmonella Typhimurium in the ceca of supplemented chickens. As indicated by Skanseng et al., 2010, a combination of 1.5% formic acid and 0.1% sorbic acid significantly reduced Campylobacterjejunicolonization in broiler chickens. According to this report when the concentration of formic acid was increased to 2% along with 0.1% sorbic acid, it completely prevented C. jejuni colonization in birds.
It has been argued that effectiveness of SCFAs as butyric depends on properties such as concentration, stability in feed manufacturing (salts less effected by temperature), palatability and smell, speed of release as ideally small intestine is the main target of release to help villi growth (salts vs. coated), and antimicrobial effects in large intestine. SCFAs are reported more effective against microorganisms at a lower pH, since the concentration of undissociated molecules of the fatty acids are greater at lower pH especially below their pKa.
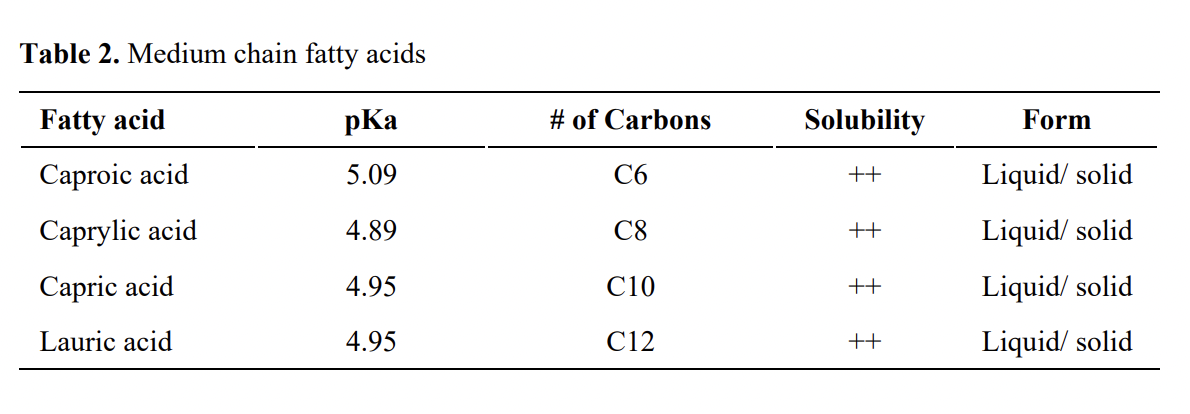
MCFAs (Table 1) namely caproic (C6), caprylic (C8), capric (C10), and lauric (C12) fatty acids are naturally found in coconut oil, palm kernel oil, and milk. Antibacterial effects of MCFAs on gram-positive and gram- negative microorganisms is well documented. Studies targeting major food-borne pathogens, Salmonella and Campylobacter are limited. However, it is reported that MCFAs are generally more inhibitory against Salmonella than SCFAs. According to Kollanoor Johny and Venkitanarayanan, 2012, caprylic acid (50 mM and 100 mM) inclusion at 0.7% and 1% reduces S. Enteritidis in the cecum of broilers by ~ 1.5 and 3 log10 cfu/mL. In the same study, similar dose dependent reductions in S. Enteritidis colonization were observed in liver, spleen, intestine, crop and cloaca of chickens. Overall, studies by Kollanoor Johny suggested that caprylic acid could reduce significant numbers of Salmonella in tissue samples including ceca of chickens.
Solis de los Santos et al. (2009) reported that 0.7% caprylic acid reduced (2 to 3 log10 cfu/g) C. jejuni colonization consistently in market-age (42-day) broiler chickens when supplemented during last 3 to 7 days of the trial after infecting them with the pathogen on day 21. Authors did not observe significant changes in body weight, feed intake or cecal pH in birds. In addition, they reported that although C. jejuni counts were reduced with caprylic acid supplementation, endogenous cecal bacterial counts did not differ significantly between caprylic acid supplemented groups and controls. Interestingly, when 0.7% caprylic acid was supplemented for last 3 days of the trial, C.jejunicolonization was reduced by 3 log10 cfu/g, even after a 12 hour feed withdrawal period. Feed withdrawal before slaughter is a common poultry industry practice, it has been associated with increased microbial contamination due to pecking of manure- contaminated litter. The overall results suggest that caprylic acid at 0.7% concentration when supplemented through feed could reduce C. jejuni colonization in commercial broiler chickens prophylactically and therapeutically, and can be even used to reduce pathogen carriage during feed withdrawal periods.
Administration of MCFAs through drinking water remains a possibility. Reviewing the literature, it seems caprylic acid administration via water provided variable results in chickens. In one study 0.175% caprylic acid reduced cecal C. jejuni counts by ~ 3 log10 cfu/g. At higher concentrations of caprylic acid (≥ 1.4%), feed and water consumption and body weight of birds were significantly reduced. MCFA-mediated bacterial reduction in chickens is discussed in the literature and several hypotheses are suggested for it. It has been showed by Molatova et al. (2009) that after treatment with capric acid C. jejuni cells were damaged extensively; however there was no change in cellular permeability. Another report suggested mechanism is by changing the cecal microbial population by caprylic acid.
Fatty acids and protozoal challenge
Few species of Eimeria spp. impact poultry species e.g., broilers and turkeys with major economic losses. The role of fatty acids on controlling Eimeria spp challenge has been investigated in limited number of studies. Challenge studies on the use of n-3 fatty acids from fish oil (2.5 to 10%), expressed flax seed oil (10%) yielded promising results in reducing E. tenella-induced lesions in chickens. Yang et al. (2006) reported that supplementation of fatty acids in poultry oil (more in C16:0, C18:1 and C18:2 n-6) elevated mortality rate in chickens infected with E.tenella. However, the studies oils did not reduce the lesions in chickens caused by E. tenellainfection.
References are available on request
From the Proceedings of the MPF 2020

















