Vaccination programs for breeders and egg layers include procedures with inactivated vaccines. Killed (inactivated) vaccines are used to prevent disease in the vaccinated bird and/or to provide maternal antibody for protection of progeny. Killed vaccines are often more costly than live organism vaccines and in addition the labour cost to administer killed vaccines can be more expensive than the vaccine, both of which are important reasons to vaccinate correctly.
Even more important is that incorrect immunization exposes the vaccinated birds or their progeny to disease, with resultant welfare consequences and economic loss. Also killed vaccines can cause serious injuries to staff if accidentally injected. Hence it is important for efficacy, welfare, safety and economic reasons that killed vaccines are administered safely and correctly. Audits of Quality Control Points of vaccination with killed vaccines on 16 farms revealed considerable variability in results between farms and indicated to management where improvements in vaccination procedures should be implemented. It was recommended that killed vaccination audits should be undertaken within poultry companies as part of the Quality Control process to manage the efficacy and safety of killed vaccinations.
Introduction
Killed vaccines are included in programs of breeders and commercial egg layers. Effective immunization with killed vaccines requires: a technically sound vaccination program appropriate for the flock and the infectious disease status in the area; correct receipt, storage, transport, preparation, and administration of killed vaccines; auditing of vaccination procedures; and serological monitoring, where appropriate, to confirm that the vaccination program and procedures are likely to be effective. Quality Control audits of killed vaccination were undertaken in 16 flocks to assist poultry companies manage immunization of their flocks.
Materials and methods
Killed vaccination audits: sixteen audits of vaccination into breast muscle with killed vaccines containing antigens of infectious bursal disease (IBD), Newcastle disease (ND), egg drop syndrome (EDS), Salmonella Types B+C+E, Pasteurella multocida (fowl cholera) or Avibacterium paragallinarum (infectious coryza) were conducted over a two-year period for six of Australia’s major chicken meat companies, two audits for Australia’s two major egg layer breeder companies and an audit for one chicken meat breeder company in New Zealand. Each audit of the Australian chicken meat companies was undertaken in different regional divisions of the company throughout Australia.
Quality control procedures used for monitoring killed vaccination
- Store vaccine in an alarmed refrigerator at 2-8 ⁰ Do not freeze. Check for “separated emulsion”.
- Remove vaccine from the refrigerator the day before vaccination and hold in an air-conditioned room overnight to allow the vaccine to reach 15-25 ⁰C prior to injection or warm to 25 ⁰C in a water bath immediately before using.
- Transport vaccine from storage rooms to farms/houses without ice bricks to retain temperature at 15-25 ⁰
- Add blue dye (MSD VacTrace for bird injection) to at least one container of vaccine per vaccinator.
- Shake vaccine containers vigorously before and during use for thorough mixing.
- Calibrate the gun by injecting at least 10 doses into a syringe or measuring cylinder to confirm the correct dose of the injection.
- Use new needles every 1.000-2.000 birds.
- Observe vaccine administration to confirm that the vaccine is being injected correctly into the fleshiest part of the breast and safely to prevent “needle-stick” injuries to staff.
- Vaccinate at least 1.000 birds, including all sex errors and culls, with the blue colored vaccine to obtain 20 sex errors and culls for post-mortem examination.
- Euthanize and post-mortem the vaccinated sex errors and culls with the vaccination crew present to confirm that the vaccine has been deposited correctly into the breast muscle.
- Reconcile the number of doses used against the number of birds vaccinated.
- Discuss any improvements to the vaccination procedures that need to be implemented with the supervisor and poultry company management.
- Blood test at least 20 birds/house six weeks following vaccination for IBD, ND or EDS, as appropriate.
Results
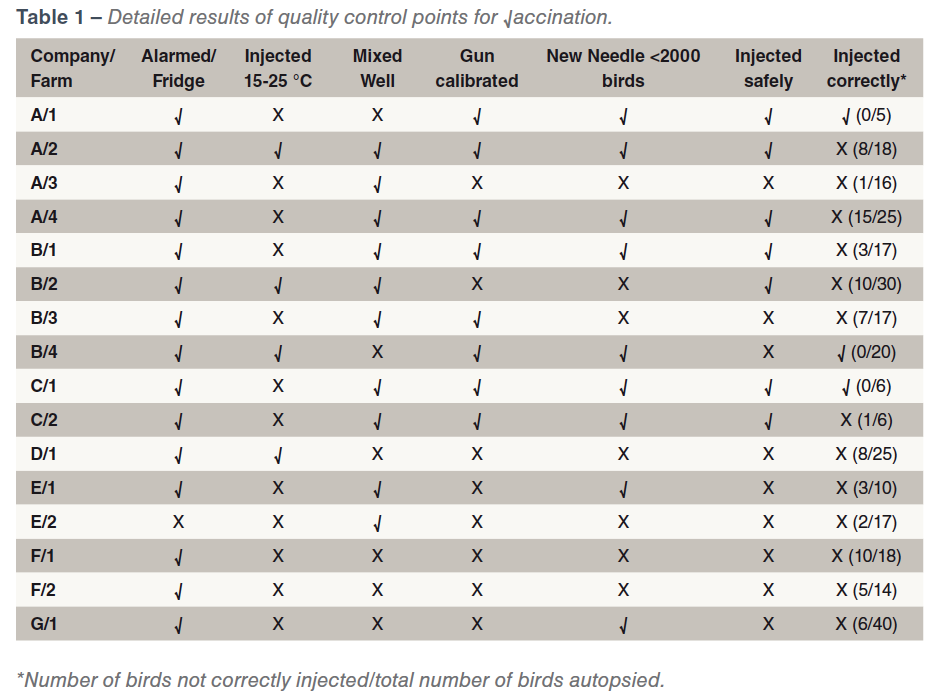
There was some variability in the standard of the vaccination procedures which could influence the effectiveness and safety of the vaccination. Vaccination crews were always interested in observing the results of their vaccinations and were often surprised at the results. Storage of vaccine was generally satisfactory. Only one farm did not have an alarmed refrigerator. Eleven farms did not warm vaccine to 15-25 ⁰C, as per label instructions, to prevent cold shock to the bird, to prevent intense local reactions and to reduce viscosity of the vaccine to ensure an even flow through the vaccination gun needle thus generating a full dose for each bird to optimise the effectiveness of the vaccine. Six farms did not mix the vaccine sufficiently to ensure that each bird is injected with a full dose of vaccine. Eight farms did not calibrate the vaccination gun. Seven farms did not change needles frequently, with resultant bruising and leakage of vaccine onto the breast muscle surface or into feathers in some cases. Nine farms did not adequately protect against “needle stick” injuries to staff. Thirteen farms did not vaccinate all birds correctly into the breast muscle. A summary of the results is given in Table 1.
Serological monitoring
Relevant serological monitoring was either not undertaken or the results were not available. Achievable mean ELISA titers for IBD virus are >12.000 with a CV <20% at 4 to 6 weeks after killed vaccination in chicken meat breeders to provide protection of progeny against infection with Australian standard or Australian variant IBD viruses that can cause immunosuppression in the first two weeks of life. At 4 to 6 weeks following killed vaccination of breeders and egg layers, achievable mean HI titers for ND are >8 (log 2) and for EDS >6 (log 2).
Discussion
Auditing killed vaccination, as described in this paper, revealed that there were deficiencies in vaccination procedures which could result in lack of efficacy in vaccinated birds or protection of progeny against immunosuppression and which could jeopardize the safety of staff. Interestingly, vaccination crews usually considered that they were vaccinating effectively and safely and were very surprised when audits showed that this was not the case. Killed vaccinations are an integral component of vaccination programs for long-lived chickens and as such can have a major influence on the performance or liveability of the vaccinated birds or their progeny.
Correct procedures are essential for flock health, bird welfare and profitable poultry production. The audits undertaken in Australasia are also applicable in other countries, with likely resultant improvements in killed vaccination effectiveness and flock performance.
Source: Proceedings of the Sixty-seven the Western Poultry Disease Conference