
A pilot study was undertaken to assess the compatibility of a new multistrain probiotic with a live-attenuated Salmonella Typhimurium (ST) vaccine. Experimental units were individual birds. There were three experimental treatments: control group without ST-vaccine administration or probiotic in the diet (T1), ST vaccinated group without probiotic (T2) and ST vaccinated group with probiotic in the diet (T3). On day 3 of test chicks were challenged with 107 CFU/ml Salmonella Heidelberg by oral gavage. Prior to challenge, four chicks from each group were randomly selected and the vaccine was able to be re-isolated from the spleens from treatments receiving the vaccine, indicating the probiotic did not affect the vaccine’s initial colonization. At study termination (day 39) caeca were sampled for Salmonella prevalence and number. There was no significant difference in Salmonella prevalence among experimental treatments. However, the vaccine alone or with probiotic showed numerically lower MPN log10 of Salmonella than the challenge control.
The basis for effective control of Salmonella infections in poultry production is good farm management and hygienic practices as well as testing and elimination of positive flocks in some countries. While many different measures have been recommended in meat chicken farms, vaccination with live-attenuated vaccines is likely to have a central role in the reduction of Salmonella in commercial operations by increasing the passive immunity of birds and blocking the horizontal transmission of Salmonella. The advantage of live-attenuated vaccines is that attenuated Salmonella bacteria replicate, colonize, and invade intestinal and visceral organs of inoculated chickens, producing long-lasting protective immunity. Furthermore, as part of measures against Salmonella in poultry, it has been demonstrated that the addition of Bacillus-based probiotics in poultry feed may result in significant reductions of Salmonella load in the intestinal tract of chickens as well as in the surrounding environment, thereby potentially reducing the risk of infection between birds and decreasing the amount of Salmonella entering the slaughterhouse, thus potentially improving food safety. The objective of this pilot study was to evaluate the effect of a Bacillus-based probiotic on the colonization of a live Salmonella Typhimurium (ST) vaccine and its subsequent ability to protect against a Salmonella Heidelberg challenge in broiler chickens.
One hundred and twenty (120) day-of-hatch Ross x Ross non-sexed broiler chicks were obtained from a commercial broiler company (Georgia, US). At the experimental facility (Southern Poultry Research Group, Inc. -SPRG-, Georgia, US), birds were randomly distributed in three isolated room divisions (40 birds per division), with each room division assigned to one of the following experimental treatments: control group without ST-vaccine administration or probiotic in the diet (T1), ST vaccinated group without probiotic (T2) and ST vaccinated group with probiotic in the diet (T3). All birds were vaccinated with an approved broiler coccidiosis vaccine, 30 minutes before administrating live ST-vaccine to T2 and T3 birds. The ST-vaccine was coarse sprayed, as per manufacturer’s recommendation, at one day of age at one dose per bird in a volume of 0.25 ml per chick.
The birds were raised under thermostatically controlled gas heaters and ambient humidity and were provided a lighting program as per the primary breeder recommendations. At placement, each isolated division contained approximately 10 cm of fresh pine shavings. Litter was not replaced during the course of the study. Each isolated division contained one tube feeder and one bell drinker resulting in a forty bird/feeder and drinker ratio. Feed and water were administered ad libitum. Since performance assessment was not an aim of the study, only two feeding phases were established: starter phase from day zero to day 21, grower-finisher phase from 22 to day 39. Diets were fed as crumbles (starter phase) or pellets (grower-finisher phase). Feed formulations for this study consisted of un-medicated commercial-type broiler starter and grower diets compounded with commonly used United States feedstuffs representative of local formulations, calculated analyses to meet or exceed NRC standards. No antibiotics were added to any feed. Treatment feeds were prepared from a basal starter feed with quantities of all basal feed to prepare treatment batches documented. Treatment feeds were mixed at the SPRG feed mill and pelleted in a California Pellet mill at 80 °C. Starter feed and grower-finisher feed for T3 birds was supplemented with a multi- strain probiotic composed of the following probiotic strains: Bacillus subtilis DSM32324 (8 x 105 CFU / g of feed), Bacillus subtilis DSM32325 (5 x 105 CFU / g of feed) and Bacillus amyloliquafaciens DSM25840 (3 x 105 CFU / g of feed). Thus, the total content of supplemented Bacillus strains in T3 feed was 1.6 x 106 CFU / g of feed.
On the third day of life, all birds were orally gavaged with a 3 x 107 CFU nalidixic acid-resistant Salmonella Heidelberg. Prior to challenge, four ceca and four spleens from each treatment were weighed and collected to confirm vaccine colonization. The samples were analyzed by decimal dilutions in buffered saline with gelatine. The diluted samples were plated in drops on Salmonella-Shigella agar and incubated at 37 °C for 24 h (permitting detection of 102 CFU of salmonellae per g of sample). The presence or absence of salmonellae at concentrations below 102 CFU/g in the sample was tested by ability or inability to detect salmonellae in samples incubated in selenite cysteine broth at 37 °C for 48 h, sub-cultured on Salmonella-Shigella agar, and incubated for 24 h at 37 °C. Samples positive by selective enrichment in selenite cysteine broth were recorded as 10 CFU, and negative samples were recorded as 0 CFU.
On day 39, 10 birds per treatment were taken from each isolated division, euthanised (by cervical dislocation), and caeca aseptically removed. After removal, each caecal sample was placed in one sterile plastic sample bag (Fisher Scientific), labeled and stored on ice and transferred to the onsite laboratory for Salmonella analysis. The caeca samples were weighed, sterile saline was added to them, and they were stomached. An aliquot of 1 ml was removed for Most Probable Number (MPN) procedure.
For all 10 caeca, a sample of 1 ml of stomached peptone broth was transferred to three adjacent wells in the first row of a 96-well 2 ml deep block. An aliquot of 0.1 ml of sample was transferred to 0.9 ml of tetrathionate broth in the second row. This process was repeated for the remaining rows in order to produce five ten-fold dilutions. Blocks were incubated (24 hours at 42 °C). and 1 μl of each well transferred onto XLT-4 agar (containing nalidixic acid) with a pin-tool replicator. Plates were incubated at 37 °C for 24 hours, the final dilution of each sample being recorded and entered in MPN calculator (to determine sample MPN).
Suspect Salmonella isolates were confirmed by Poly-O Salmonella Specific Antiserum (MiraVista, Indianapolis, IN), according to Berghaus et al. (2013) and Alali et al. (2013).
Salmonella prevalence was compared between treatment groups using Fisher’s exact test. Salmonella MPNs in culture-positive samples were compared between treatment groups using one-way analysis of variance. MPN values were log-transformed prior to statistical analysis. Post-hoc pairwise comparisons were performed using the Bonferroni procedure to limit the type I error probability to 5% over all comparisons. All tests assumed a two-sided alternative hypothesis, and P < 0.05 was considered statistically significant. Analyses were performed using commercially available statistical software (Stata 15.0, StataCorp LLP, College Station, TX). Although statistical comparisons were performed for informational purposes, they are not valid under the pilot study design because birds within the same room division (pen) are not statistically independent, and there was no pen-level replication of treatments.
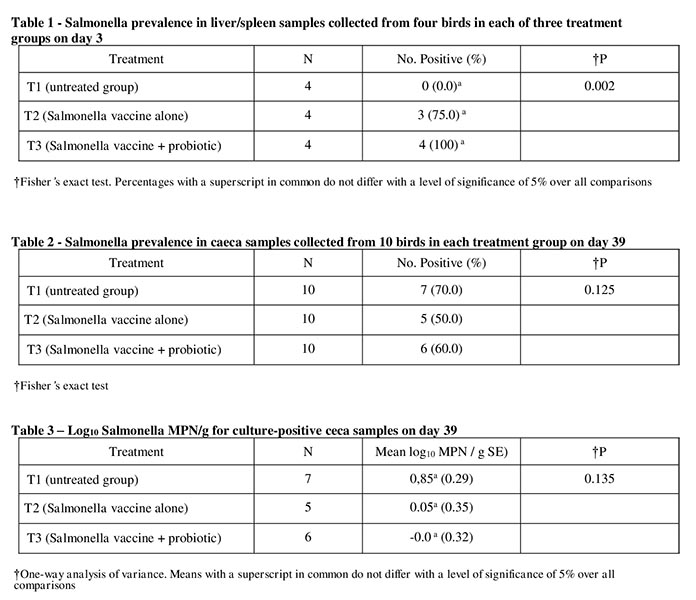
Liver and spleen samples were cultured from four birds per treatment group on day 3. Salmonella prevalence is summarized in Table 1. There was a significant overall treatment effect with respect to Salmonella prevalence (P = 0.002). Prevalence in T1 was zero, whereas in T2 and T3 was 75% and 100%, respectively, indicating that probiotic supplementation in the diet did not interfere with the response to the vaccine. Due to the small sample sizes, none of the individual pairwise comparisons between treatments were significant when using the Bonferroni procedure to limit the type I error probability to 5% over all comparisons. All of the Salmonella isolates obtained on day 3 were identified as belonging to serogroup B, which was consistent with the vaccine strain (Salmonella Typhimurium).
Salmonella prevalence in ceca samples at day 39, based on MPN data, is summarized in Table 2. There was no significant difference between treatments (P = 0.125). All of the Salmonella isolates obtained from caeca samples were identified as belonging to serogroup B. As nalidixic acid was used in the MPN procedure, all isolates obtained were presumed to be the challenge strain. Although there was not a significant reduction, there was a consistent numerical reduction in prevalence of the challenge strain in both treatments.
Salmonella MPNs for the culture-positive caeca samples are summarized in Table 3. There was no significant difference between treatments with respect to the mean log10 Salmonella MPN / g in culture-positive caeca samples on day 39 (P = 0.135), although the application of the live-attenuated vaccine alone or with probiotic resulted in lower values than the untreated group.
This study was primarily designed to demonstrate that the tested probiotic would have no negative effect on the ability of the live Salmonella vaccine to replicate and induce protection. This study clearly demonstrated that this Bacillus-multistrain probiotic does not have any detrimental effect. IReferences are available on request
From the Proceedings of the 2018 Australian Poultry Science Symposium
















