
Histomoniasis, commonly known as blackhead disease, is caused by an anerobic protozoan parasite, Histomonas meleagridis. Histomoniasis affects all gallinaceous birds and turkeys are the most vulnerable species. In recent years, increased incidences of histomoniasis have been documented in chickens.
Vijay Durairaj1, Mary Drozd2, Emily Barber1, Brandon Doss3, Ryan Vander Veen1
1 Huvepharma, Inc., Lincoln, Nebraska, USA
2 University of Nebraska-Lincoln, Lincoln, Nebraska, USA
3 Huvepharma, Inc., Peachtree City, Georgia, USA
Corresponding author: Vijay.Durairaj@huvepharma.us
A case report of histomoniasis in a four-week-old-broiler breeder pullet farm is discussed here. On field investigation, gross lesions in ceca and liver were noticed suggesting histomoniasis. During histopathological evaluation, Histomonas trophozoites were identified. H. meleagridis and Blastocystis spp. were isolated in culture. Advanced molecular diagnostic techniques confirmed genotype-1 H. meleagridis. With the absence of commercial vaccines and prophylactic/therapeutic measures, field surveillance studies play a vital role in understanding H. meleagridis wild-type strains and identifying solutions for histomoniasis.
Introduction
Histomoniasis was first reported in turkeys by Cushman (1893). Histomonas meleagridis, an anerobic protozoan parasite, causes histomoniasis (histomonosis, enterohepatitis, enzootic typhlohepatitis). H. meleagridis affects all gallinaceous birds and turkeys are highly susceptible. Heterakis gallinarum, the common cecal worm of chickens, acts as a vector for transmitting H. meleagridis. Heterakis gallinarum eggs can harbor H. meleagridis for several years. Thus, it is not advisable to rear chickens and turkeys on the same premise or in close proximity to one another.
Among the gallinaceous species, turkeys are highly susceptible to histomoniasis. In turkeys, the mortalities can reach up to 80%-100% (Hess, 2020). Chickens mount a better immune response to H. meleagridis compared to turkeys (Powell et al., 2009; Mitra et al., 2017). Thus, the severity of histomoniasis is not as pronounced in chickens and may induce mortality up to 10%-20% (McDougald, 2005). Based on 1986 AAAP Committee on Disease Reporting, the economic losses associated with histomoniasis are more significant in chickens compared to turkeys due to the number of birds involved and frequency of incidences (Callait, 2002). In recent years, increased incidences of histomoniasis have been documented in chickens.
In the past, histomoniasis was managed and controlled by prophylactic treatment with Arsenics (i.e Carbarsone and Nitarsone), and therapeutic treatment with Nitro-imidazoles (Dimetridazole, Iprondidazole) and Nitrofurans (Furazolidone, Salfuride) (Clark, 2017). Due to various reasons, these products were removed/withdrawn from the market. At present, there are no commercial vaccines available to combat histomoniasis. In certain countries, a few therapeutic/ prophylactic products are used to combat histomoniasis, but the efficacy of these products are highly variable.
With this set of circumstances, the prevention of histomoniasis transmission by following strict biosecurity measures helps in minimizing the risk of spreading the disease. Field surveillance studies aid in understanding the current H. meleagridis wild-type isolates and to identity potential solutions for histomoniasis.
Materials and methods
Case history
In Spring 2022, histomoniasis was reported in two out of seven houses (n=14,000 birds/house) in four-week-old-broiler breeder pullets in South Central, USA. Increased mortality was reported in both houses. On necropsy, gross lesions were noticed in the ceca and liver. Ceca and liver samples were collected in 10% neutral buffered formalin for histopathological evaluation. Additional cecal samples were collected in plug seal-capped flasks containing 10 ml of modified Dwyer’s media (Hauck, 2010) and placed in a warm insulated Styrofoam box and safely transported to the lab. In addition, whole intestines were collected and shipped in a cold insulated Styrofoam box.
Histopathology
Necropsy tissues were immediately fixed in 10% neutral buffered formalin. Following fixation, sections were processed routinely, paraffin-embedded and sectioned at 3-4 μm and stained with hematoxylin-eosin. Histologic tissues were evaluated by a board-certified pathologist.
Culture
The plug seal-capped flasks with cecal samples were received in warm condition. The flasks were supplemented with fresh pre-warmed modified Dwyer’s media (5 ml) and then moved to an incubator (40°C) and maintained in anaerobic condition. Periodically, the cultures were observed under inverted microscope.
DNA extraction and PCR
Intestinal samples were evaluated and a piece of cecal tissue was homogenized with glass beads. DNA was extracted from the homogenized cecal sample using DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. Each 50 µL PCR reaction consisted of 1X GoTaq G2 Hot Start Green Master Mix (Promega, Madison, WI), 0.2 µM of each primer, and 5 µL of template. PCR was performed using protozoa 18s rRNA primers (9. Bilic 2014), H. meleagridis rpb1 gene specific primers (9. Bilic 2014), SSU rRNA of Blastocystis primers (10. Hess et al., 2006), and mtCOI gene of Eimeria sp. primers along with the described cycling conditions.
Gel electrophoresis of PCR products and sequencing
The amplicons (2 µL) were visualized using E-Gel™ EX Agarose Gels, 2% (Invitrogen, Carlsbad, CA) with E-Gel™ 1 Kb Plus DNA Ladder (Invitrogen™). The QIAquick PCR purification kit (Qiagen) was used to purify the PCR products for sequencing (Eurofins, Louisville, KY).
Results
Gross pathology
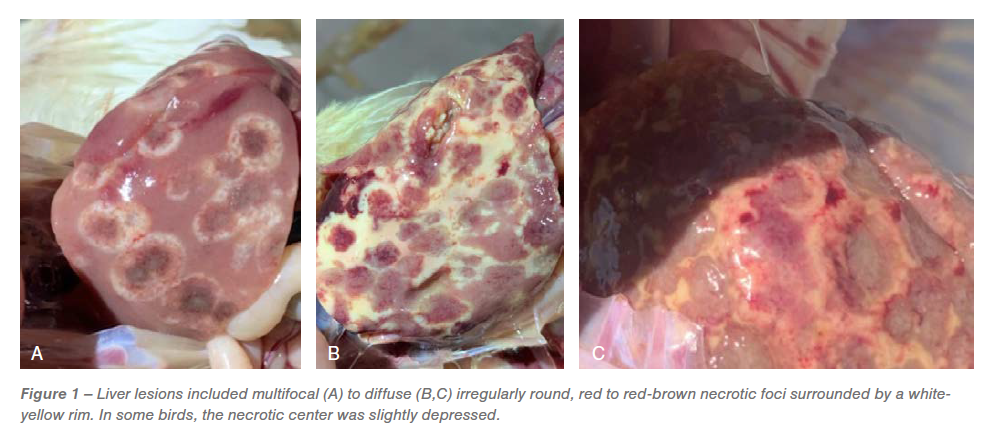
Gross lesions in the ceca included typhlitis and cecal cores. The liver lesions had numerous dark red centered multifocal necrotic foci surrounded by white periphery resembling bulls-eye (Figure 1A), diffuse irregularly round, red necrotic foci surrounded by white-yellow rim, resulting in discoloration of the liver (Figure 1B) and red-brown necrotic foci resulting in slight depressions on the surface of the liver resembling saucer shaped lesions (Figure 1C). Based on the distinctive gross lesions in the ceca and liver, a presumptive diagnosis of histomoniasis was made.
 Histopathology
Histopathology
Ceca: Approximately 60% to 95% of the mucosa was replaced by predominately granulomatous inflammation and granulation tissue infiltrated by pleomorphic bacteria and Histomonas trophozoites (Figures 2 and 3). The luminal surface was covered in a fibrinocellular exudate infiltrated by protozoa and bacteria. The underlying submucosa was effaced by histiocytic to pyogranulomatous inflammation and granulation tissue that is infiltrated by trophozoites (Figures 2 and 3). 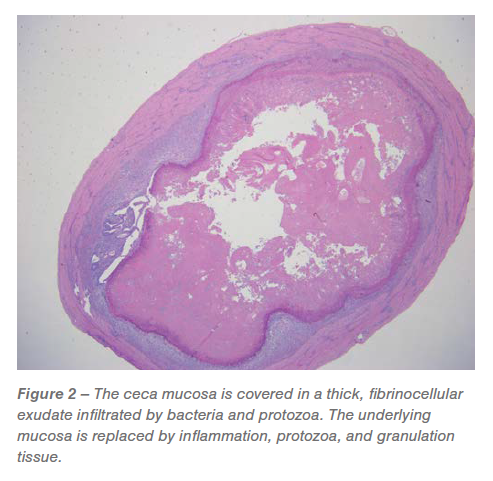
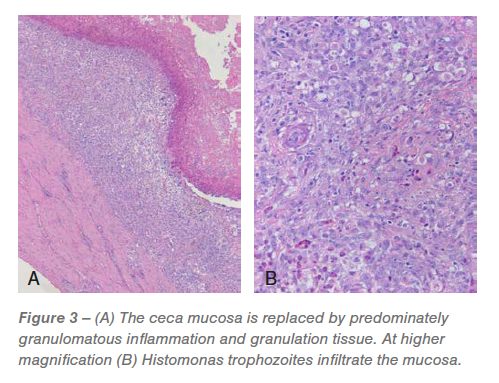
The remaining mucosa had abundant lamina propria fusion and inflammation, bacterial and protozoa, crypt loss and extensive, irregular distention of the remaining crypts with protein and cellular debris. The tunica muscularis was expanded and mildly effaced by multifocal to coalescing, predominantly histiocytic inflammation that is most prevalent around small blood vessels and occasionally has a central nidus of necrosis and trophozoites. The associated mesentery was thickened by lymphohistiocytic inflammation.
Liver: Between 20% and 75% of the examined liver sections were severely effaced by multifocal to coalescing, random, hepatocellular necrosis infiltrated by histiocytic and mixed inflammation with granuloma organization, variable numbers of trophozoites and protein (Figure 4). 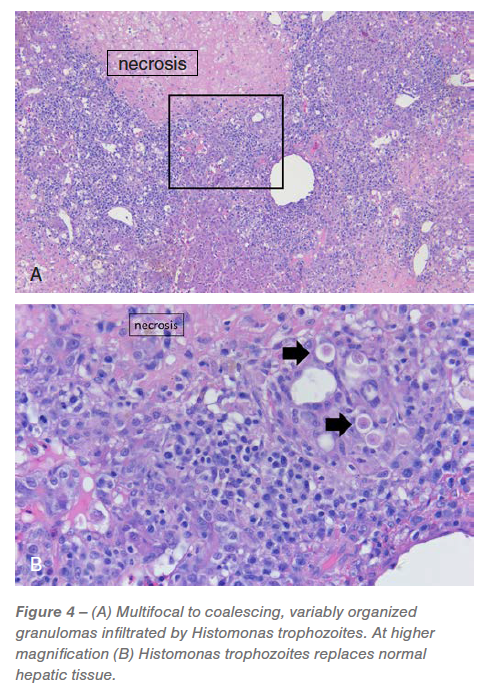
Culture
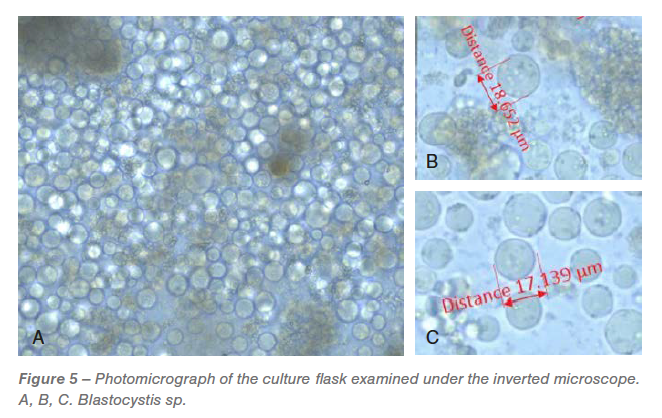
On the day after receipt, all the flasks were examined under the microscope. An overwhelmed Blastocystis spp. population (Figure 5) with varying sizes were observed in all the flasks. Two days after incubation, H. meleagridis were detected in the culture but were difficult to document by microscopic images due to overwhelming growth of Blastocystis spp. All the flasks had unidentified bacterial population.
 PCR and sequencing
PCR and sequencing
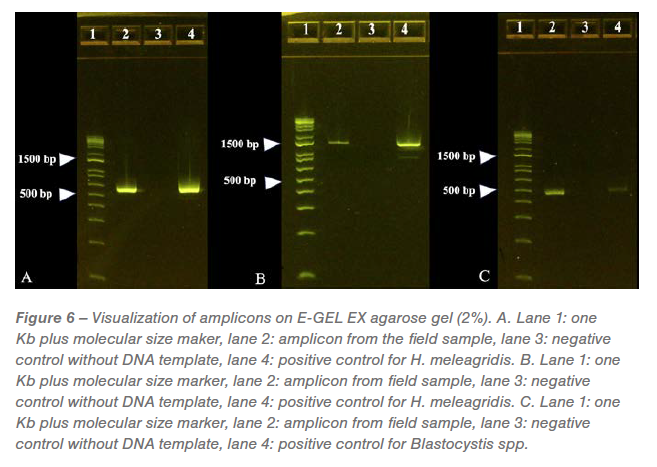
The predicted size of each amplicon generated from the PCR reactions was visualized on separate E-Gels. A positive band was noticed at approximately 550 bp in the amplicons generated by PCR directed against protozoal 18s RNA and confirmed as genotype-1 H. meleagridis with 95.17% identity to wild-type H. meleagridis (Figure 6A). A positive band was noticed at approximately 1240 bp in the amplicons generated by PCR directed against H. meleagridis Rpb1 gene and confirmed as genotype-1 H. meleagridis with 99.66% identity to wild-type H. meleagridis (Figure 6B). A positive band was noticed at approximately 500 bp in the amplicons generated by PCR directed against Blastocystis spp. with 99.30% identity to wild-type Blastocystis spp. (Figure 6C). No bands were observed on Eimeria sp. specific PCR (gel image not shown).
 Discussion
Discussion
Histomoniasis in chickens was infrequently reported in the past. Withdrawal and ban of prophylactics/therapeutics in poultry has resulted in increased cases of histomoniasis. The number of birds involved, along with increased incidences and accompanying morbidity and mortality, has positioned histomoniasis as an economically important disease in the chicken industry. In the USA, a day-old broiler breeder female chick costs ~ $10 and male chick costs ~$14. Considering the feeding, management and labor costs at four-weeks-of-age, a broiler breeder pullet costs approximately $13 to $17. For example, in a flock of 14,000 birds at four-weeks-of-age, if 1% of increased mortality is attributable to histomoniasis, it will result in additional losses of $1820-$2380. If the mortality associated with histomoniasis increased to 5%, it will result in an additional loss of $9,100 to $11,900.
A presumptive diagnosis of histomoniasis was made based on the characteristic gross lesions in the ceca and liver. In this case, the liver lesions were distinctive suggesting histomoniasis. In some instances, H. meleagridis does not cause typical lesions in the liver. In other instances, the liver and cecal lesions may be induced by different pathogens. Lesions in the chicken liver can be induced by parasites such as H. meleagridis, Tetratrichomonas gallinarum and Leucocytozoon caulleryi, viruses such as Marek’s disease virus, Avian leukosis virus, reticuloendothelial virus, fowl adenovirus-1, avian hepatitis E virus and bacteria such as Salmonella pullorum, Salmonella gallinarum, Pasteurella multocida, Mycobacterium avian, Enterococcus cecorum, Streptococcus gallolyticus subsp. gallolyticus, Escherichia coli (perihepatitis), Mycoplasma gallisepticum (perihepatitis), Mycoplasma synoviae (perihepatitis), Clostridium perfringens, Camplylobacter hepaticus, Staphylococcus, and Erysipelothrix rhusiopathiae. Other conditions that can cause liver lesions include hemorrhagic hepatopathy, fatty liver hemorrhagic syndrome, aflatoxins, amyloidosis, ascites, visceral gout, heat stress and high energy diet. Lesions in the chicken ceca can be induced by parasites such as H. meleagridis, Eimeria tenella, Tetratrichomonas gallinarum and bacteria such as Salmonella sp. Combined liver and cecal lesions in chickens can be induced by H. meleagridis, Tetratrichomonas gallinarum and Salmonella Sp.
Histology of the ceca and liver confirmed H. meleagridis as the cause of necroulcerative typhilitis and necrotizing and granulomatous hepatitis in these chickens.
The cecal culture incubated in modified Dwyer’s media had overwhelming growth of Blastocystis spp. of various sizes. Blastocystis spp. is a common protozoan parasite that is present in the intestine of the chickens (Grabensteiner et al., 2006; Chadwick et al., 2000). Although debatable, Blastocystis sp. does not have a huge clinical impact (Chadwick et al., 2000; Stensvold et al., 2009).
H. meleagridis has two genotypes, namely genotype 1 and 2 (Bilic et al., 2014). The pathological manifestations and the clinical signs vary between the genotypes. PCR and sequencing confirmed that the wild-type H. meleagridis reported in this study was genotype 1. In Europe, genotype 1 is predominant, while genotype 2 is rare (Bilic et al., 2014). In the USA, based on our field surveillance over the last few years, genotype 1 was identified in all the field outbreaks studied. Advanced molecular techniques such as PCR and sequencing helps provide valuable insights at the genotype level. Thus, field surveillance studies help in understanding current H. meleagridis wild-type isolates and can be useful in identifying a solution for histomoniasis.
Acknowledgment
The authors would like to thank the poultry company and the supervisor for collaborating in this field case.
References
- Cushman, S. The production of turkeys. In: Bulletin 25, Agricultural Experiment Station, Rhode Island College of Agriculture and Mechanical Arts, Kingston, RI. 89–123. 1893.
- Hess M., McDougald LR. Histomoniasis. In: Swayne D, Boulianne M, Logue C, McDougald L, Nair V., Suarez D., deWit S., Grimes T., Johnson D., Kromm M., et al., editors. Diseases of Poultry. 14th ed. Ames (IA): Wiley- Blackwell. 1223–1230; 2020.
- Powell, F. L., L. Rothwell, M. J. Clarkson, and P. Kaiser. The turkey, compared to the chicken, fails to mount an effective early immune response to Histomonas meleagridis in the gut. Parasite Immunol. 31:312–327. 2009.
- Mitra, T., W. Gerner, F. A. Kidane, P. Wernsdorf, M. Hess, A. Saalmuller, and D. Liebhart. Vaccination against histomonosis limits pronounced changes of B cells and T-cell subsets in turkeys and chickens. Vaccine 35:4184–4196. 2017.
- McDougald LR. Blackhead disease (histomoniasis) in poultry: a critical review. Avian Dis. 49:462–476; 2005.
- Callait, M. P., C. Granier, C. Chauve, and L. Zenner. In vitro activity of therapeutic drugs against Histomonas meleagridis (Smith, 1895). Poult. Sci. 81:1122–1127. 2002.
- Clark S, Kimminau E. Critical review: future control of blackhead disease (histomoniasis) in poultry. Avian Dis. 61:281–288; 2017.
- Hauck R, Armstrong PL, McDougald LR. Histomonas meleagridis (Protozoa: Trichomonadidae): analysis of growth requirements in vitro. J Parasitol. 96:1–7; 2010.
- Bilic I, Jaskulska B, Souillard R, Liebhart D, Hess M. Multi-locus typing of Histomonas meleagridis isolates demonstrates the existence of two different genotypes. PLOS ONE 9:e92438; 2014.
- Hess, M., T. Kolbe, E. Grabensteiner, and H. Prosl. Clonal cultures of Histomonas meleagridis, Tetratrichomonas gallinarum and a Blastocystis sp. established through micromanipulation. Parasitology. 133:547–54. 2006.
- Grabensteiner, E., and M. Hess. PCR for the identification and differentiation of Histomonas meleagridis, Tetratrichomonas gallinarum and Blastocystis spp. Vet. Parasitol. 142:223–230. 2006.
- Chadwick E, Malheiros R, Oviedo E, Cordova Noboa HA, Quintana Ospina GA, Alfaro Wisaquillo MC, Sigmon C, Beckstead R. Early infection with Histomonas meleagridis has limited effects on broiler breeder hens’ growth and egg production and quality. Poult Sci. 99:4242-4248. 2020.
- Stensvold CR, Alfellani MA, Nørskov-Lauritsen S, Prip K, Victory EL, Maddox C, Nielsen HV, Clark CG. Subtype distribution of Blastocystis isolates from synanthropic and zoo animals and identification of a new subtype. Int J Parasitol. 39:473-9. 2009.

















