
Cecal coccidiosis is an important disease that affects the cecal integrity of turkeys and may result in watery feces with flecks of blood. In turkeys it is caused by three Eimeria species.
Turkeys have a pair of cecal pouches located at the junction of the ileum and colon. The ceca act as fermentation chamber and plays a role in fermentation of carbohydrates. It also helps in the digestion of crude protein, fiber, cholesterol, degradation of nitrogen compounds, and reabsorption of water, electrolytes and cholesterol (1). The turkey cecal pH is around 5.9 (1). Within a few days after hatch, turkey ceca can harbor around 10¹¹ organisms per gram of digesta (2). The cecal microbiota is complex and includes several microbiota including bacteria, viruses, archaea, fungi, and protozoa (3). Any insult to the ceca can affect the cecal health, which in turn adversely affects the homeostasis of the cecal contents.
Cecal droppings are dark in color (Figure 1A) and the consistency of cecal droppings is different from rectal feces. The rectal droppings are green, brown or gray with a white cap representing urates (Figure 1B).
 A compromise in cecal health may be attributable to pathogens, feed quality, or toxins. Among the pathogens affecting cecal health, protozoan infections, especially Eimeria infections, are commonly seen due to the ubiquitous presence in the intense poultry raising operations. Eimeria in turkeys was first documented by Theobald Smith in 1895 (4).
A compromise in cecal health may be attributable to pathogens, feed quality, or toxins. Among the pathogens affecting cecal health, protozoan infections, especially Eimeria infections, are commonly seen due to the ubiquitous presence in the intense poultry raising operations. Eimeria in turkeys was first documented by Theobald Smith in 1895 (4).
Cecal coccidiosis is an important disease that affects the cecal integrity of turkeys and may result in watery feces with flecks of blood (Figure 1C). Cecal coccidiosis in turkeys is caused by three predominant Eimeria species:
1. E. adenoeides– highly pathogenic
2. E. gallopavonis– highly pathogenic
3. E. meleagridis– non-pathogenic.
E. adenoeides
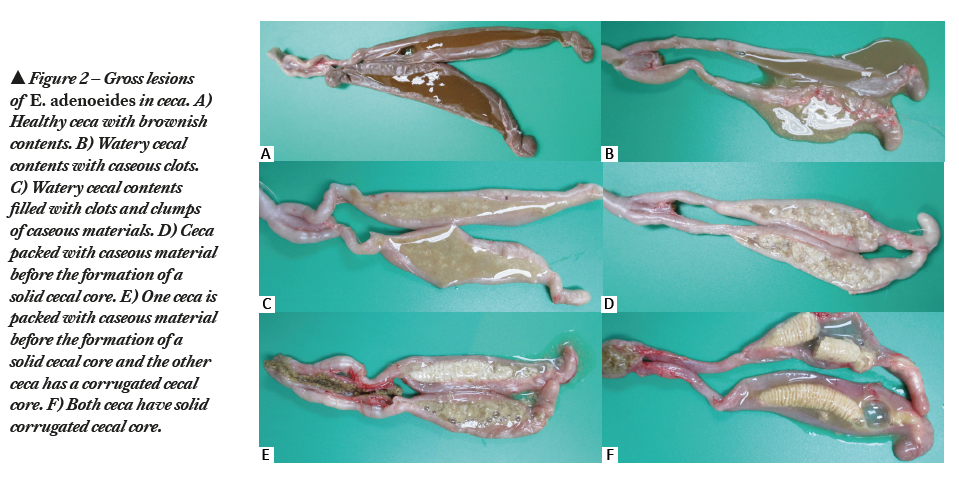
E. adenoeides was first reported by Moore and Brown in 1951 (5). Being a highly pathogenic Eimeria species (6), E. adenoeides is one of the predominating species documented in USA (7). The prepatent period of E. adenoeides is 4.3 days (103 hours). E. adenoeides targets ceca and causes petechiae, congestion, edema, necrosis and sloughing of the cecal mucosa. Pathological manifestation of E. adenoeides includes watery cecal contents with cheesy caseous clots (Figure 2B, 2C, 2D), partially formed cecal core (Figure 2E) or solid cecal core (Figure 2F). In severe cases, the lesions may extend up to the lower small intestine and the cloaca. Infection causes dehydration, decreased feed intake, poor feed conversion, decreased body weight gain, and watery feces. In severe cases, it causes blood-tinged diarrhea along with mucous casts and mortalities.
E. gallopavonis
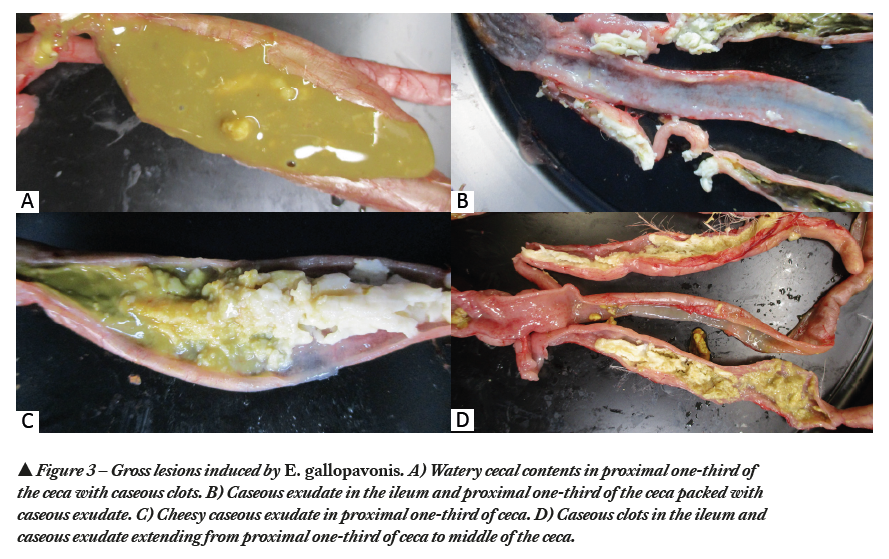
E. gallopavonis was first reported by Hawkins in 1950 (8). Being a highly pathogenic Eimeria species (6), E. gallopavonis is one of the predominating species documented in USA (7). The prepatent period of E. gallopavonis is 4.4 days (105 hours). E. gallopavonis targets primarily ileo-cecal junction, extends up to cecal neck and rectum and causes petechiae, ulceration, hemorrhage and thickening of the intestinal wall. Pathological manifestation of E. gallopavonis includes formation of caseous clots or chunks (Figure 3A) in the cecal contents and presence of cheesy exudate in the anterior cecal neck (Figure 3B, 3C, 3D). Infection causes dehydration, decreased feed intake, poor feed conversion and decreased body weight gain. In severe cases, it causes flecks of blood in the feces and mortalities.
E. meleagridis
E. meleagridis was first reported in 1927 by Tyzzer (9). E. meleagridis is considered as a nonpathogenic species (5). Infecting poults with a very high number of oocysts neither induced clinical symptoms nor weight loss (5). A slight drop in body weight was documented by Hawkins in 1952 (10). The prepatent period of E. meleagridis is 4.5 days (110 hours). Several studies characterized E. meleagridis as nonpathogenic with very minimal damage (11). Due to very minimal damage, it is often unnoticed in flocks.
Conclusion
Ensuring cecal health helps in improving the gut health. Several intervention strategies are available to control cecal coccidiosis in turkeys, including vaccines, synthetic compounds/chemicals, ionophores, and natural alternative products.
References
1. Proszkowiec-Weglarz M. Gastrointestinal anatomy and physiology. In: Scanes CG, Dridi S, editors. Sturkie’s avian physiology. 7th ed. London (U.K.): Academic Press. p. 485–527; 2022.
2. Apajalahti J, Kettunen A, Graham H. Characteristics of the gastrointestinal microbial communities with special reference to the chicken. World Poult Sci J. 60:223–32. doi:10.1079/WPS20040017; 2004.
3. Wei S, Morrison M, Yu Z. Bacterial census of poultry intestinal microbiome. Poult Sci. 92:671–683; 2013.
4. Smith, T. Investigations concerning infectious diseases among poultry Bulletin No. 8 (pp. 7–27). Washington, DC: United States Department of Agriculture, Bureau of Animal Industry; 1895.
5. Moore, E.N. & Brown, J.A. A new coccidium pathogenic for turkeys, Eimeria adenoeides n. sp. (Protozoa: Eimeriidae). The Cornell Veterinarian. 41, 124–135; 1951.
6. Lund, E.E. & Farr, M.M. Coccidiosis of the turkey. In H.E.Biester & L.H. Schwarte (Eds.), Diseases of Poultry 5th edn (pp.1088–1093). Ames, IA: Iowa State University Press. 1965.
7. Duff AF, Briggs WN, Bielke JC, McGovern KE, Trombetta M, Abdullah H, Bielke LR, Chasser KM. PCR identification and prevalence of Eimeria species in commercial turkey flocks of the Midwestern United States. Poult Sci.101(9):101995; 2022.
8. Hawkins, P.A. Coccidiosis of the turkey. Journal of Parasitology, 36 (2, Suppl.), 42–43; 1950.
9. Tyzzer, E. E. Species and strains of coccidia in poultry. J. Parasitol. 13:215; 1927.
10. Hawkins, P.A. Coccidiosis in Turkeys. Technical Bulletin 226. East Lansing, MI: Michigan State College Agricultural Experiment Station; 1952.
11. Cervantes HM, McDougald, LR, Jenkins MC. Coccidiosis. In: Swayne D, Boulianne, M, Logue C, McDougald L, Nair V, Suarez D, deWit S, Grimes T, Johnson D, Kromm M, et al., editors. Diseases of poultry. 14th ed. Ames (IA): Wiley-Blackwell. p. 1193–1216; 2020.

















