
Part 1
The first part in the series of two wich analyses the anthropological impact of the chicken from an archaeological/historical point of view: broilers had a role in economic development (indigenous and improved breeds in rural or pastoralist production, the change to industrial and larger scale peri-urban production is likely to be a major driver of meeting food requirements).
Changing patterns of human resource use and food consumption have profoundly impacted the Earth’s biosphere. Until now, no individual taxa have been suggested as distinct and characteristic new morphospecies representing this change. Here we show that the domestic broiler chicken is one such potential marker. Human-directed changes in breeding, diet and farming practices demonstrate at least a doubling in body size from the late medieval period to the present in domesticated chickens, and an up to fivefold increase in body mass since the mid-twentieth century. Moreover, the skeletal morphology, pathology, bone geochemistry and genetics of modern broilers are demonstrably different to those of their ancestors. Physical and numerical changes to chickens in the second half of the twentieth century, i.e. during the putative Anthropocene Epoch, have been the most dramatic, with large increases in individual bird growth rate and population sizes. Broiler chickens, now unable to survive without human intervention, have a combined mass exceeding that of all other birds on Earth; this novel morphotype symbolizes the unprecedented human reconfiguration of the Earth’s biosphere.
- Background
Accelerating human-driven physical, chemical and biological changes to the Earth system have been profound, sparking suggestions of a new geological epoch, the Anthropocene. Human consumption trends have driven unprecedented changes to the Earth’s biosphere. Populations of wild animal groups have plummeted in recent decades, while human and livestock populations have risen. The biomass of humans and their domesticated animals (including livestock) now outweighs that of all wild terrestrial vertebrates. Understanding the nature of this change is important to help protect biodiversity. Domesticated chickens (Gallus gallus domesticus Linnaeus, 1758) are a striking example of a human reconfigured biosphere. They are the world’s most numerous bird with a standing population of 22.7 billion. This population is an order of magnitude greater than the standing stocks of the most abundant wild bird species (red-billed quelea approximately 1.5 billion, house sparrow approximately 0.5 billion, rock dove approximately 0.25 billion), other farmed birds (turkeys approximately 0.3 billion, geese approximately 0.3 billion, ducks approximately 1.1 billion) and farmed pigs and cattle (figure 1). In Europe, the population of domesticated chickens in 2009 (1.9 billion) was greater than the combined population of the 144 most numerous wild bird species (1.6 billion). It is likely to be the largest standing population of a single bird species in Earth’s history.
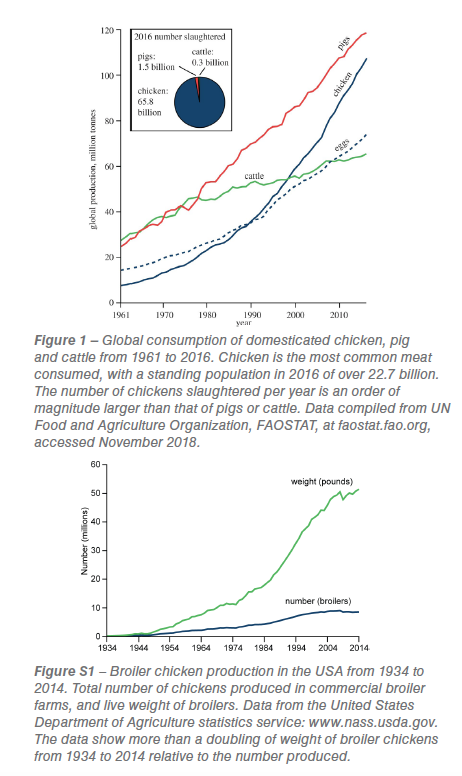
Chicken-meat consumption is growing faster than any other meat type and is soon to outpace pork (figure 1). Expanding consumption in developing countries is driving the trend. This has had a profound impact on the biology of the broiler (meat-chicken). Since the Chicken-of-Tomorrow Program in the early 1950s, launched to encourage the development of higher meat-yielding birds, chickens have undergone extraordinary changes. From the mid-twentieth century to the present, broiler growth rates have soared, with up to a fivefold increase in individual biomass. Here, we document the biology of the broiler from the Roman era to the present and discuss whether the biological changes to the broiler are distinctive enough to make them a marker species of the proposed Anthropocene Epoch. Changes to the broiler skeleton, diet and genetics (preserved as geochemical signatures in their bones) have the potential to be fossilized.
The current dominance of Gallus gallus domesticus as the world’s most numerous bird species would not be possible without modern technology. The system of industrial chicken production and its export around the world has facilitated surging chicken-meat consumption. Separate broiler breeding units, farms, slaughterhouses, processing plants and marketing are coordinated into one system called vertical integration. First implemented in the southern USA in the 1950s, vertical integration systems now account for 97% of USA broiler production. Industrial chicken farming is now widespread around the globe (figure 2) and has enabled the rapid rise in broiler production from the 1950s onwards (figure 1; figure S1). In 2006 it was estimated that 70% of broilers were intensively reared. The system epitomizes efficient and high volume production from a human perspective: in the USA the top broiler production firm, Tyson Foods, slaughtered 35 million chickens per average week in 2012. Retailers of chicken are some of the most successful and globalized, for example KFC, the world’s largest chicken meat retailer, has over 25.500 stores in 125 countries worldwide (www.kfc.com, accessed February 2018).
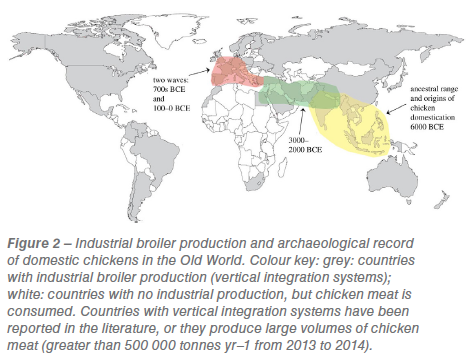
The domestic chicken originated from the red jungle fowl (Gallus gallus Linnaeus, 1758), native to tropical S/SE Asia. Contemporary, non-indigenous red jungle fowl occur in the Americas, Australia, Europe and Africa, due to deliberate human translocation, and are adapted to a much wider climatic range than indigenous birds. The fossil record of the red jungle fowl is poorly known, but molecular-clock estimates place the origin of the order Galliformes (pheasants, jungle fowl and relatives) in the early Cretaceous. However, the archaeological record of chicken domestication and husbandry is relatively well documented (figure 2). Archaeological studies had suggested that chickens were domesticated ca 8000 years ago, although ongoing DNA and dating research is likely to bring this date forward. Domesticated chicken bones are recorded from the Indus valley as early as 2500–2100 BCE. The time-transgressive spread of domesticated chickens out of Asia is contemporaneous with the establishment of new trade routes. For example, chickens were introduced to the Iberian Peninsula by Phoenician traders in the first century CE and Spanish colonists introduced domesticated chickens to the New World in 1500 CE.
- Methods
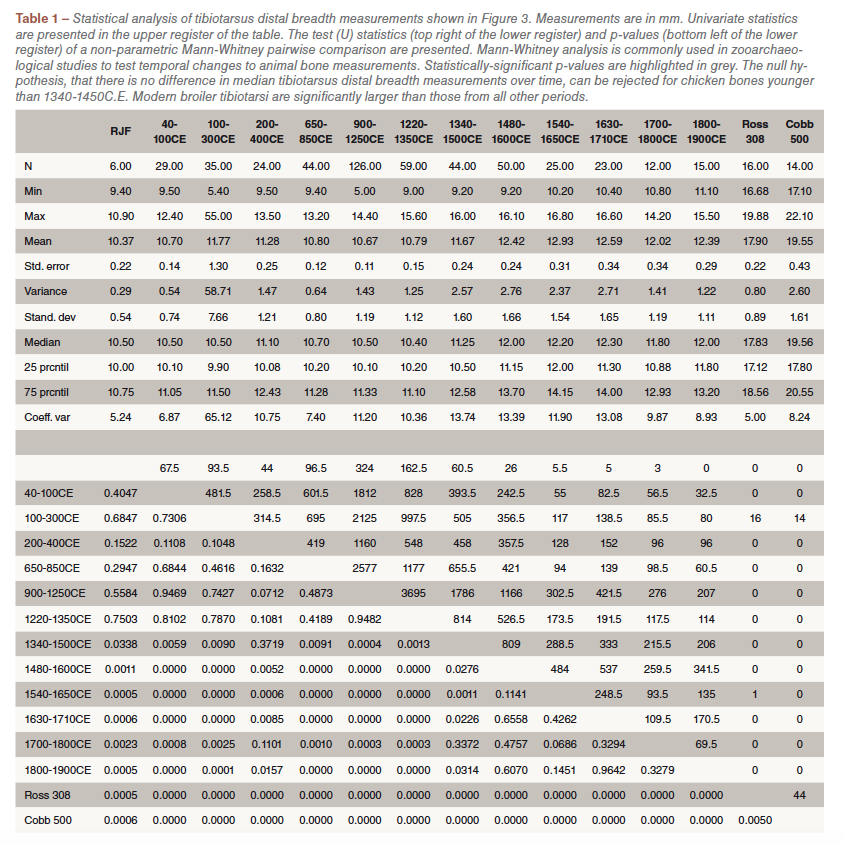
Since the early 1990s the Museum of London Archaeology (formally the Museum of London Specialist Services and Museum of London Archaeological Service) has systematically recorded zooarchaeological data from developer-funded excavations in London. Included among this dataset is an enormous archive of animal bone measurements taken using the standard set out by von den Driesch (1976). In this study, 486 individual tibiotarsus distal breadth measurements from 74 sites from the city were analysed and compared with modern broiler data. The distal width was measured in preference to length of the tibiotarsus because the majority of bone specimens were broken. To avoid potential confusion with age-related size change, measurements from archaeological specimens were all derived from skeletally mature birds. To facilitate the identification of temporal trends and accommodate the majority of the archaeological data, bone measurements were placed into 12 overlapping temporal groups (table S1). Assemblages that were broadly or insecurely dated assemblages (i.e. bones from contexts with residual pottery) were excluded from analysis. It is possible that the group of domestic fowl bones include guinea fowl (Numida meleagris L., 1758) and pheasant (Phasianus colchicus L., 1758) bones, which are morphologically similar to chicken. This is pertinent to the archaeological record of early chicken domestication: the re-examination of early Holocene bones from northern China reputed to provide early evidence of chicken domestication, revealed that most of the bones were from pheasants rather than chickens. However, pheasant and guinea fowl are only identified rarely so it is assumed that most fowl bones derive from chicken. A non-parametric Mann–Whitney pairwise comparison was run using the statistical software PAST to examine the statistical significance of broiler skeletal size (table S1).
Data on broiler and other livestock populations was sourced from the FAO (Food and Agriculture Organization of the United Nations) website (http://faostat3.fao.org) and the United States Department of Agriculture statistics service (www.nass.usda.gov). Data on chicken growth rates, osteo-pathologies and bone collagen isotopes were synthesized from numerous publications (cited in the figure captions).
- Results
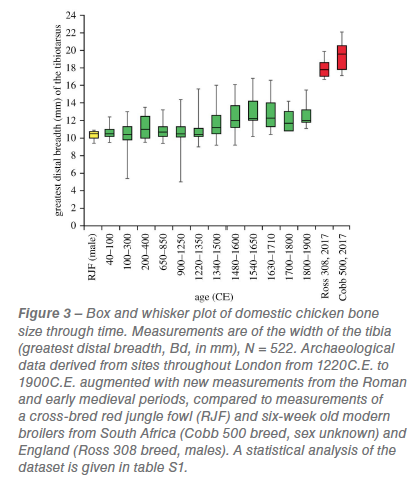 The size of chicken bones from multiple archaeological sites in London, UK, is recorded from the Roman era to the end of the nineteenth century (figure 3) compared against a red jungle fowl and two broiler datasets. Modern broiler skeletons are significantly larger than both the wild progenitor bird and archaeological domestic chickens (figure 3; table S1). The greatest distal breadth of the tibiotarsus (which provides a proxy of body mass) is in some specimens twice the width in a six-week-old modern broiler than in an adult red jungle fowl (figure 3). More dramatic, is a direct comparison
The size of chicken bones from multiple archaeological sites in London, UK, is recorded from the Roman era to the end of the nineteenth century (figure 3) compared against a red jungle fowl and two broiler datasets. Modern broiler skeletons are significantly larger than both the wild progenitor bird and archaeological domestic chickens (figure 3; table S1). The greatest distal breadth of the tibiotarsus (which provides a proxy of body mass) is in some specimens twice the width in a six-week-old modern broiler than in an adult red jungle fowl (figure 3). More dramatic, is a direct comparison 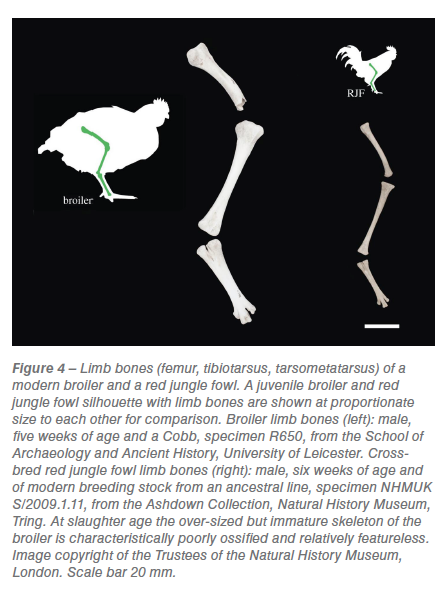 of juvenile broiler and red jungle fowl lower limb bones of the same age, which shows a tripling in width and a doubling in length (figure 4). From the Roman era until 1340 the distal widths of domesticated chicken tibiotarsi in London were similar to those of their red jungle fowl ancestor (table S1). Sustained increases in the size of chickens from 1340 to 1650 (figure 3) are concurrent with size increases in other domesticated livestock. The average distal tibiotarsus width measurements from 1650 up until 1900 remain fairly constant (figure 3).
of juvenile broiler and red jungle fowl lower limb bones of the same age, which shows a tripling in width and a doubling in length (figure 4). From the Roman era until 1340 the distal widths of domesticated chicken tibiotarsi in London were similar to those of their red jungle fowl ancestor (table S1). Sustained increases in the size of chickens from 1340 to 1650 (figure 3) are concurrent with size increases in other domesticated livestock. The average distal tibiotarsus width measurements from 1650 up until 1900 remain fairly constant (figure 3).
While the archaeological record for chickens indicates alterations in bone morphology related to domestication, the speed and scale of changes escalated considerably in the second half of the twentieth century. A surge in chicken production from the 1950s onwards (figure 1) 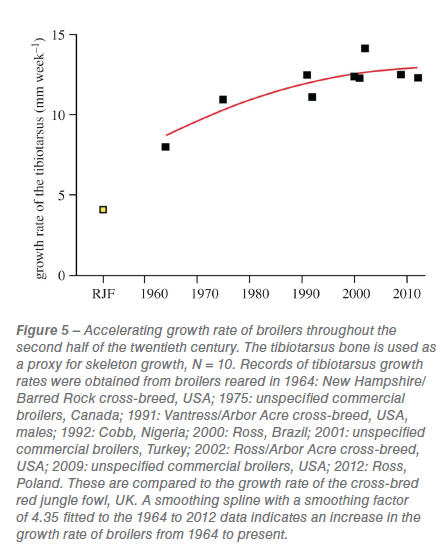 has resulted in an increase over this period in the mass of individual birds (figure S1). We quantify how the growth rate of broilers (from juvenile to adult) has changed over the twentieth century by a compilation of data from several commercial breeds (figure 5; figure S1). There has been a steady increase in growth rate since 1964 and the growth rate of modern broilers is now three times higher than that of the red jungle fowl. However, data from the twenty-first century show that the growth rate is slowing (figure 5; figure S1) and may be reaching a plateau.
has resulted in an increase over this period in the mass of individual birds (figure S1). We quantify how the growth rate of broilers (from juvenile to adult) has changed over the twentieth century by a compilation of data from several commercial breeds (figure 5; figure S1). There has been a steady increase in growth rate since 1964 and the growth rate of modern broilers is now three times higher than that of the red jungle fowl. However, data from the twenty-first century show that the growth rate is slowing (figure 5; figure S1) and may be reaching a plateau.
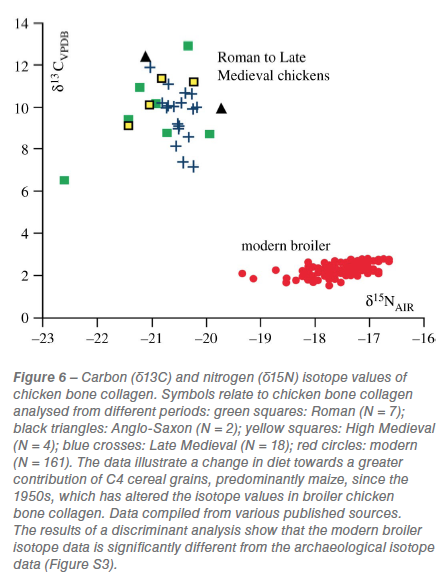
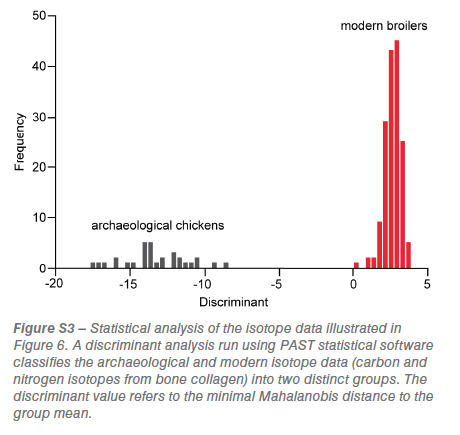
A change in the diet of domesticated chickens to produce high meat-yields is detected in the analysis of dietary carbon (δ13C) and nitrogen (δ15C) isotopes (figure 6). Chicken bone collagen isotopes from the Roman, Anglo-Saxon, High Medieval and Late Medieval periods are distinct from those of modern broilers (figure 6; figure S3).
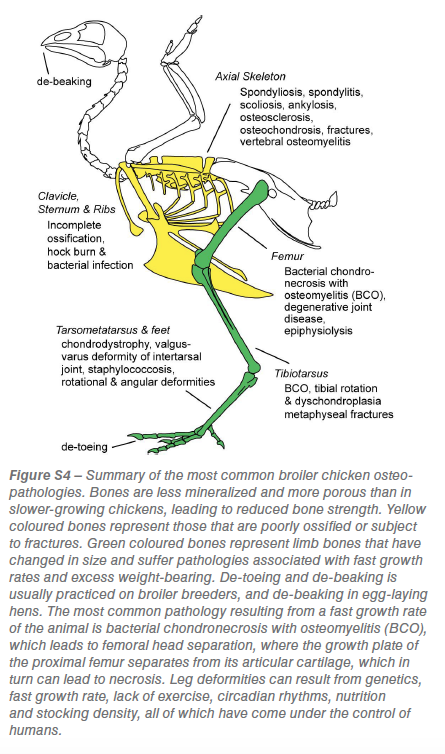
 Multiple and significant osteo-pathologies arise as a consequence of the accelerated growth rate of broilers (figure S4). These pathologies are common in broilers, but are only rarely observed in archaeological bones: for example, tibial dyschondroplasia (poor ossification of the tibia leading to lameness) is recorded in a turkey from nineteenth-century London, but has never been reported in chicken bones from archaeological contexts.
Multiple and significant osteo-pathologies arise as a consequence of the accelerated growth rate of broilers (figure S4). These pathologies are common in broilers, but are only rarely observed in archaeological bones: for example, tibial dyschondroplasia (poor ossification of the tibia leading to lameness) is recorded in a turkey from nineteenth-century London, but has never been reported in chicken bones from archaeological contexts.
End of the first part
References are available on request
Read the second part
Footnotes
Electronic supplementary material is available online at https://dx.doi.org/10.6084/m9.figshare.c.4305458.
© 2018 The Authors.
Published by the Royal Society under the terms of the Creative Commons Attribution License http://creativecommons.org/licenses/by/4.0/, which permits unrestricted use, provided the original author and source are credited.















