
The GIT, or rather the gastro intestinal tract, is the organ of the all organism mostly colonized by the microbial population: it hosts more than 70% of all the microbes of the animal body and forms the gastro intestinal microbiome, consisting of bacteria, yeasts, fungi and viruses.
The viruses present, quantitatively represent the largest number of gastro intestinal microbiome composition, excluding the presence of viruses responsible for malabsorption syndrome, the viral population is represented almost exclusively by bacteriophages or phages.
The discovery of bacteriophages dates back to the late 1800s, precisely in 1896, when the British bacteriologist Ernest Hanbury Hankin observed that the waters of the river Ganges and the river Jumna, in India, possessed antibacterial properties that had reduced the incidence of cholera and dysentery cases in areas located near the two rivers. Hankin hypothesized that these antibacterial properties were to be attributed to an unknown substance, able to go beyond the porcelain filters used to filter the river water, but which could degrade at high temperatures (thermolabile). Nearly twenty years after Hankin’s first observations, another British bacteriologist named Frederick Twort observed a phenomenon similar to that described by Hankin and hypothesized that “the unknown substance” with antibacterial activity could be a virus. However, due to the lack of funds, Twort could not continue his research in this area.
It was only two years later that the French-Canadian microbiologist Felix d’Herelle, in the early 1900s, discovered or better rediscovered, the existence of bacteriophage viruses. Shortly after his discovery, d’Herelle made the first attempt to introduce bacteriophage therapy for the treatment of dysentery. This attempt, had positive results. Thereafter, bacteriophage therapy was also tested for the treatment of other infections and, even in these cases, positive results were obtained. However, with the advent of antibiotics, in the West the use of bacteriophages was shelved in favour of newly discovered drugs; in Eastern Europe, on the other hand, therapy with bacteriophages has continued to develop and is still used today.
Viruses, and bacteriophages, are viruses, unlike bacteria, they are not able to replicate themselves, they are obligatory parasites, just because they have to exploit other cells to reproduce. When the exploited cell is an animal one, the viruses create a damage to the host organism; when to be exploited, it is a pathogenic bacterial cell for the animal, the bacteriophages can become allies of the health, (the enemy of my enemies is my friend) and they could become even more in the near future, considering the growing problem of drug resistance and the consequent bacterial insensitivity not only to antibiotics, but also to other molecules such as essential oils, up to copper (sulphate) and zinc (oxide).
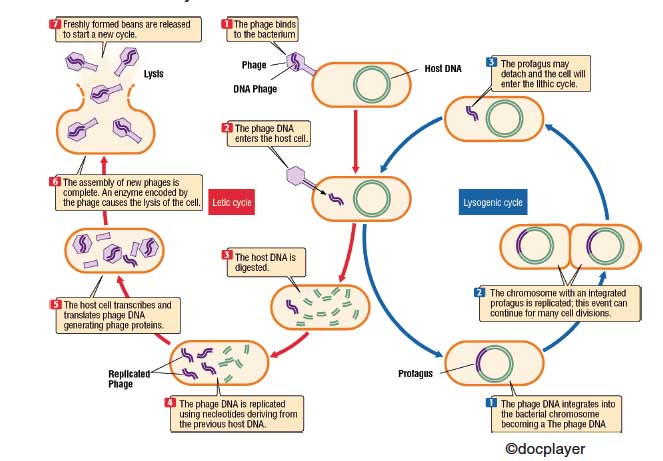
 A bacteriophage or phage (Figure 1) is a virus that parasites a particular bacterium, pathogenic or non-pathogenic, which can cause destruction by lysis. The bacteriophage attacks the bacterium by fixing the fibers on a precise point on the surface of the bacterium. With a contraction mechanism injects its nucleic acid, while the protein envelope remains outside. Once injected, the phage genome can follow two ways:
A bacteriophage or phage (Figure 1) is a virus that parasites a particular bacterium, pathogenic or non-pathogenic, which can cause destruction by lysis. The bacteriophage attacks the bacterium by fixing the fibers on a precise point on the surface of the bacterium. With a contraction mechanism injects its nucleic acid, while the protein envelope remains outside. Once injected, the phage genome can follow two ways:
A) In the lithic cycle (typical of the T bacteriophages), it will use the replication apparatus of the host to produce new phage particles, until reaching the burst volume, at which time the cell will disintegrate by lysis.
B) In the lysogenic cycle, instead, the phage genome is integrated into a specific point of the bacterial chromosome. In this integrated state, the phage is called the profagus, and whenever the bacterial chromosome replicates, the integrated genome also replicates. A bacterium that contains a profagus is called “lysogenic”. The state of profagus is maintained by a specific protein produced by the phage itself. The removal of this repressor induces the lithic cycle.
 Bacteriophages can be distinguished into two main categories, depending on whether their genome has the ability to fit into the bacterial chromosome, managing to replicate with it. In fact, some bacteriophages, once the infection is completed, always determine the production of new virions, while others only exceptionally lead to lysis the infected bacterium. The bacteriophages of the first type are called virulent; the seconds are called temperate bacteriophages.
Bacteriophages can be distinguished into two main categories, depending on whether their genome has the ability to fit into the bacterial chromosome, managing to replicate with it. In fact, some bacteriophages, once the infection is completed, always determine the production of new virions, while others only exceptionally lead to lysis the infected bacterium. The bacteriophages of the first type are called virulent; the seconds are called temperate bacteriophages.
Bacteria that carry a temperate bacteriophage integrated into its genome are called lysogens and have some differences with their progenitors, for example they are no longer sensitive to the temperate bacteriophage that has infected them, nor, at times, to bacteriophages related to that specific (lysogenic immunity). On the same basis occurs the phenomenon of conversion, whereby the non-toxigenic strains of Corynebacterium diphterie, Streptococcus pyogenes, and also other species, acquire the ability to produce toxin, if rendered lysogenic by the infection of bacteriophages from toxigenic strains.
Bacteriophages are considered to be natural enemies of bacteria and have advantages over antibiotics:
1) They are highly specific, as each bacteriophage is selective for a given bacterial species, or even for specific bacterial strains. This characteristic is very important because, in this way, the bacteria responsible for the infection are the only targets of the therapy and we do not witness, as often happens with antibiotics, the indiscriminate killing of the bacteria that form the whole convenient bacterial flora; all this results in a reduction in side effects caused by many antibiotics, such as diarrhoea and hypovitaminosis.
2) Thanks to the lysis of bacteria, bacteriophages indirectly stimulate the immune system. In fact, bacterial lysis generates cell fragments that are recognized by the immune system, activating the response.
3) It may happen that the bacteria develop resistance also towards the bacteriophages, but if this happens, the phages are able to develop in a short time a new strain able to attack the bacteria again.
4) When the bacteria responsible for the infection are eliminated, even the bacteriophages disappear.
5) They are cheap, as they are easily available in nature.
 Despite these advantages, however, the bacteriophages may also present some disadvantages:
Despite these advantages, however, the bacteriophages may also present some disadvantages:
1) Since bacteriophages are highly specific to certain types of bacteria, the preparation based on phages must be customized for each intervention. It is necessary to identify exactly the bacteria responsible for the infection. This can cause problems when there is an urgent need to intervene quickly in cases of high morbidity and mortality.
2) Bacterial toxins harmful to the animal may be contained within the phage preparation.
3) The transfer of bacterial genes between one virus strain and the other can occur, this can be avoided by not using lysogenic loop beans.
4) Following the bacterial lysis, excessive amounts of toxins can be released in the organism and cause a toxic shock, this obstacle can be overcome through genetic engineering techniques, with the aim of depriving the phages of the genes necessary for the synthesis of lysine; this also limits the proliferation of phages, as without lysine, viruses replicated within the bacterial cell can not escape.
In any case, despite the disadvantages, bacteriophage therapy remains a valid alternative to antibiotic therapy, as the animal organism is used to being in constant contact with phages, which are found everywhere.
The greatest obstacle is represented by the development modalities, of the phage preparations, which do not meet the Western norms that regulate the development and use of new drugs.
In the United States, however, the Food and Drug Administration (FDA) has approved the use of bacteriophage preparations for the decontamination of animals, plants and their derivatives intended for human consumption, in particular with the use of specific phages for Salmonella and Escherichia coli, in Europe the so-called “rotation” phage has allowed the dairy industry to solve the problem caused by bacteriophages of lactic bacteria, in Italy at the laboratory of probiogenomics of the University of Parma, coordinated by Prof. Ventura, have been isolated a new group of bacteriophages active against bifid bacteria, named for this bifidobatteriofagi, being the bifidobacteria the first colonizers of the GIT microbiome, this has allowed us to discover how these phages are able to regulate their development.
Regarding the situation of the antibiotic-resistance, more and more worrying than we think. Today, disease-causing bacteria adapt to antibiotics faster than the industry can generate new drugs to counteract them, creating so-called super-resistant, multi-drug-resistant bacteria that pose a serious threat to public health. Studies on how intestinal bacteria adapt to antibiotics have always turned their attention to the bacteria themselves. Lately the research has turned its attention to the phages, as they are very abundant in the intestine and are very good at ferrying the genes of one bacterium to another.
In an experimental trial, conducted by Jim Collins of Boston University (Published in Nature, June 2017), guinea pigs were treated with Ciprofloxacin or Ampicillin, two commonly used antibiotics. After eight weeks, all the viruses were collected in the guinea faeces and identified the viral genes present, comparing them with a large database of known genes. It was thus discovered that the phages treated with Ampicillin had produced more genes that help the bacteria to fight ampicillin and related drugs such as penicillin; while the phage beans treated with Ciprofloxacin had produced more genes that help the bacteria fight Ciprofloxacin and similar drugs. When the guinea pigs are treated with some classes of drugs, it is always noted the enrichment of genes of resistance to these drugs.
The intestinal microbiota has a means to protect itself, extending the basin of antibiotic resistance, allowing the bacterium to be potentially stronger and more resistant than before. Therefore, controlling the phages that lodge in the intestine, rather than the pathogens themselves, is the path to the healing and arrest of the antibiotic resistance. We are only at the beginning of this path and AVIFENOL TN can be considered the progenitor of these new possibilities offered by the continuous results of the search. AVIFENOL TN consisting of phenols and polyphenols, polymerized, able to dissociate in the GIT, releasing molecules of a phenolic nature, assimilable at the enteric level, able to activate an entero-hepatic circle, are able to favourably modify the composition of the microbiota, acting indirectly on the phages. This capacity is linked to the production process of AVIFENOL TN polymerization, able to release molecules that can be absorbed by the intestinal mucosa, and thus perform a wide range of systemic actions: antioxidants, anti-inflammatory, immunomodulatory; but the absorption of some of these active ingredients also occurs on the part of the GIT bacteria and if these bacteria have a profagus inside them, they are able to neutralize by precipitation, just the protein produced by the lysogenic phages, so the protein it is no longer able to maintain the state of profagus, its precipitation again induces the lithic cycle and the consequent bacterial lysis, without any genetic transcription of resistance.
This extraordinary mechanism of action, highly specific, shelters the same AVIFENOL TN, from the possibility of incurring in antibiotic resistance phenomena, guaranteeing a broad effectiveness over time, also in relation to particularly aggressive and multiresistant strains.
The bactericidal activity towards multiresistant bacterial strains is explained by the mechanism of action of AVIFENOL TN: the multiresistant strains have acquired this characteristic thanks to the lysophagous bacteriophages, being able to interrupt the lysogenic cycle, through the return to the lithic phase, interrupts the process of the antibiotic-resistance; this suggests that prolonged use over time may even restore the efficacy of the antibiotics themselves, which will reacquire their antibacterial properties.

















