
Managing stress in broiler breeders has been a continual topic of discussion since the early 1980’s, but our understanding of stress and its influence on gastrointestinal health and reproductive performance in poultry has only recently received attention.
Evidence from both mammalian and ecology literature, in regards to maternal stress and developmental programming, highlights the need for further investigation into the physiology and behaviour of hens during lay; to find novel strategies to alleviate stress and in turn potentially improve welfare and production outcomes of both the hen and her progeny.
Introduction
The chicken meat industry has made tremendous production gains through advanced utilisation of genetic selection and nutritional understanding to ensure that chicken meat remains a low cost, desirable product that consumers continuously demand. The industry now requires new approaches to further advance efficiency and production, with developmental programming at the forefront of industry development. Developmental programming is defined as “alterations in the in ovo environment, induced by the maternal environment, resulting in developmental adaptations that permanently change the structure, physiology, metabolism, health and production of the offspring”.
Environmental conditions provided during gestation/egg formation, primarily involving stress and nutrition, have the capacity to contribute, enhance or disrupt programming of physiological mechanisms regulating progeny growth, health, behaviour and production. Developmental programming can be affected by environmental factors, such as stress and nutrition, which compromise the maternal environment experienced by developing offspring, resulting in permanent physiological alterations to offspring phenotype, further impacting their health and productive performance. Physiological alterations can be induced directly through altered organ development and disrupted endocrine axes development, which may be mediated through epigenetic effects, with potential trans-generational impacts. Additionally, the microbial environment housed by the mother is generating enormous interest through its potential to contribute to environmental factors influencing the maternal environment, via the brain-gut-microbiota axis and its potential influence on developmental programming mechanisms.
Progeny exposure to maternal stress during early development can change an organism’s microbiota composition, and the microbiota can alter the organism’s ability to respond to stress after birth. Recent findings from our group have demonstrated that maternal stress in broilers can have significant negative effects on progeny body weight, stress-linked behaviour, immune response and body composition. The extent to which alterations to the intestinal environment, including microbiota, affects the programming of gut-brain signalling pathways of both the hen and her progeny, is an area of great interest. Considering commercial chicken meat birds now spend ~40% of their life in ovo, the influence of breeder hen rearing practices on progeny development, especially through maternal stress, may provide a pathway to improve production aspects through nutritional manipulations of already well-established industry protocols. Thus, there is substantial opportunity to optimise breeder hen health, reproductive capacity and welfare standards, all of which will improve industry profitability, as well as community perception of the chicken meat industry.
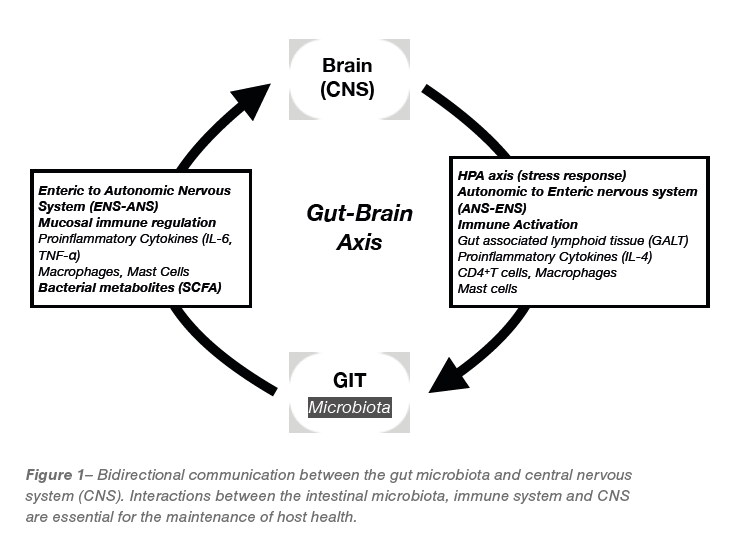
Brain-gut-microbiota axis
The intimate interaction between the central nervous system, the gastrointestinal tract and the residing microbiota, coined the brain-gut-microbiota axis has been extensively reviewed in the literature. Bi-directional communication exists between the gastrointestinal tract and the central nervous system that not only ensures the proper maintenance of gastrointestinal homeostasis and digestion but is likely to have multiple effects on higher cognitive functions. Studies on GF animals have demonstrated that the gut microbiota can modulate brain development, function and behaviour, and that brain function or behaviour can affect the microbiota composition and gastrointestinal function. Evidence supporting such modulation in chickens, however, is still elusive. A study by Calefi, et al. (2016) demonstrated that C. perfringens infection was able evoke behavioural changes in chickens; increasing the frequency of sleep-like behaviour and decreased feeding, walking, feather pecking, and standing behaviours, when birds were exposed to heat stress. Their results demonstrated a direct relationship between heat stress and C. perfringens-induced effects on gut inflammation, corticosterone serum levels and immune reactive neurons, which are related to HPA-axis activity, providing some
Communication between the brain and gut, including microbiota, occurs via complex neural (CNS, autonomic and enteric nervous system), endocrine (specifically the hypothalamo-pituitary-adrenal (HPA) axis) and immune signalling pathways, Figure 1). Disturbances to this axis, have been implicated in a wide range of disorders, including functional and inflammatory gastrointestinal disorders, such as IBD and IBS as well as extra-intestinal disorders such as allergy, obesity, cardiovascular disease and neurological disorders, such as anxiety and depression.
Gut dysbiosis
Gut dysbiosis occurs when there is a disruption in the gut-brain axis, and consequent disruption to the symbiotic relationship between gut microbial population and the intestinal mucosa. It has been speculated that intestinal inflammation develops as the result of an imbalance in the maintenance of homeostasis between intestinal commensals and immunity to pathogens, with an impairment of intestinal barrier function and increased intestinal permeability. In addition, there is also a decrease in metabolic function including the production of essential host nutrients such as short chain fatty acids (SCFA) and vitamins. Dysbiosis or dysbacteriosis has been characterised in broiler chickens with non- infectious factors, including nutritional and management stressors being key causes.
Of growing interest, is the relationship between stressor-induced alterations to the maternal brain-gut-microbiota axis, and the impact on progeny development. Primary mediators of the stress response, including corticosterone (HPA axis) and the sympathetic nervous system (SNS), can regulate multiple aspects of immune function and, in return, immune mediators trigger the stress response and modulate its effects on the gastrointestinal tract, including the growth and type of commensal and pathogenic organisms. Such axes modulation via stress factors can cause alteration to intestinal permeability, motility and changes in mucus production, causing an inflammatory response. Consequently, increased permeability of the intestinal barrier and microbial driven inflammation can then exert influence back on the HPA- axis. Continuous stress-induced impairment of the intestinal barrier creates a positive feedback- like situation whereby inflammatory cytokines persistently activate the SNS and HPA-axis resulting in barrier disruption, increased endotoxin translocation and a low-grade inflammatory state. Interestingly, this continual low-grade chronic inflammation has been reported to affect fertility and subsequent embryo development in rodent models of obesity. Investigators were able to draw correlations between obesity- mediated gut dysbiosis and markers of ovarian inflammatory responses and oocyte gene expression. Further investigation into linking stress-mediated gut dysbiosis and reproductive performance, through such mechanisms, would be of great interest not only in clinical application but also for commercial poultry production, where the health and performance of their breeding stock is paramount.
While it is becoming increasingly evident that stressor-induced alterations in the microbiota of adult animals can significantly impact host physiology and associated disorders, there is also evidence to suggest that the consequences of these alternations can have long-term negative implications in regards to both pre-natal and post-natal development of their progeny. There are two possible potential mechanisms: as mentioned before, microbial communities have been implicated in altering neuroendocrine axis, therefore changing the maternal profile of hormones and other signalling molecules that are exposed to the developing foetus and chick. Another possibility is that, in many animals, it is well established that bacterial sources for the newborn are derived from the maternal microbiota, either through the placenta, uterus and vagina, and recently discovered through the oviduct to the egg of poultry. This initial colonisation of the gut by maternally-derived microbes can ultimately “reset” neuroendocrine axes and have a profound influence on growth, health and development of progeny.
Maternal stress and progeny development
Maternal stress is well documented to influence progeny growth rate in various species and is a pressing issue in the broiler breeder industry due to current feed restriction practises. Feed restriction allows hens to maintain an optimum body weight and reproductive capacity; however it may result in hens experiencing chronic stress due to prolonged hunger with reported increase in gastrointestinal inflammation and permeability. Corticosterone depositions in the yolk of the egg have been identified, potentially providing a link between maternal stress and progeny development in egg laying species, similar to that of placental transfer in mammals.
Gestational stress in mammalian species is linked with reductions in birthweight, permanent hypertension, hyperglycaemia/hyperinsulinemia, behavioural alterations, as well as reduced immunocompetence and endocrine axis disruption. Similar physiological impacts have been noted in avian species in response to feed restriction, malnourishment and environmental conditions, such as cognitive disruption, anxiety and aggressive behaviours, delayed sexual maturation, compromised T-cell and B-cell mediated immunity and elevated baseline testosterone concentration. Decreased progeny growth rate and hatchling mass have also been reported in response to maternal stress, or in ovo administration of synthetic glucocorticoids to mimic the effects of maternal stress, although with limited consistency.
Interestingly, animal studies altering the maternal environment, either through nutritional or environmental factors, have highlighted significant phenotypic variations between male and female progeny, identifying sex-dependent changes in body composition, hormonal profiles, muscle mass and growth in response to stress. Thus, maternal stress has implications for a wide variety of physiological functions in both male and female progeny, which is immensely important when considering mixed flock performance and flock uniformity.
Studies investigating nutritional means to reduce stress in hens found that reducing caloric density but increasing dietary fibre was shown to decrease chronic hunger behaviours. On a low caloric density diet, hens were also shown to achieve significantly higher rates of lay and higher egg weights, with the percentage of fertile eggs remaining the same. The resulting progeny were heavier at hatch and 38 days old compared to progeny from hens on a standard commercial diet and also exhibited lower mortality rates. Such dietary strategies, including qualitative feed restriction, have been recently studied in broiler breeder pullets and ultimately demonstrate how improving stress and considering hen welfare could have a significant impact on progeny development, and commercial productivity.
Conclusion
Taking advantage of the potential transgenerational maternal effects within the pyramid breeding system may enable a targeted approach in regards to nutritional or other supplement interventions at significant physiological time points, such as point of lay. Optimising gut health and reproductive output though implementation of novel breeder management strategies will positively influence all facets of production, including improved behaviour, immunity, growth and efficiency of subsequent generations. However, more research is needed to disentangle the mechanisms underpinning maternal stress, gut health and developmental programming in commercial poultry production.
References are available on request
From the Proceedings of the Australian Poultry Science Symposium 2021

















