
Turkeys typically consume twice as much water as feed, so it is important to provide a clean, healthy water supply. Water not only serves as a vital nutrient but it also impacts virtually every physiological function in the body. Therefore factors which might alter water quality such as changes in bacterial content, pH, nitrogen levels, hardness, alkalinity or mineral levels might directly impact water consumption or the bird’s ability to utilise consumed water.
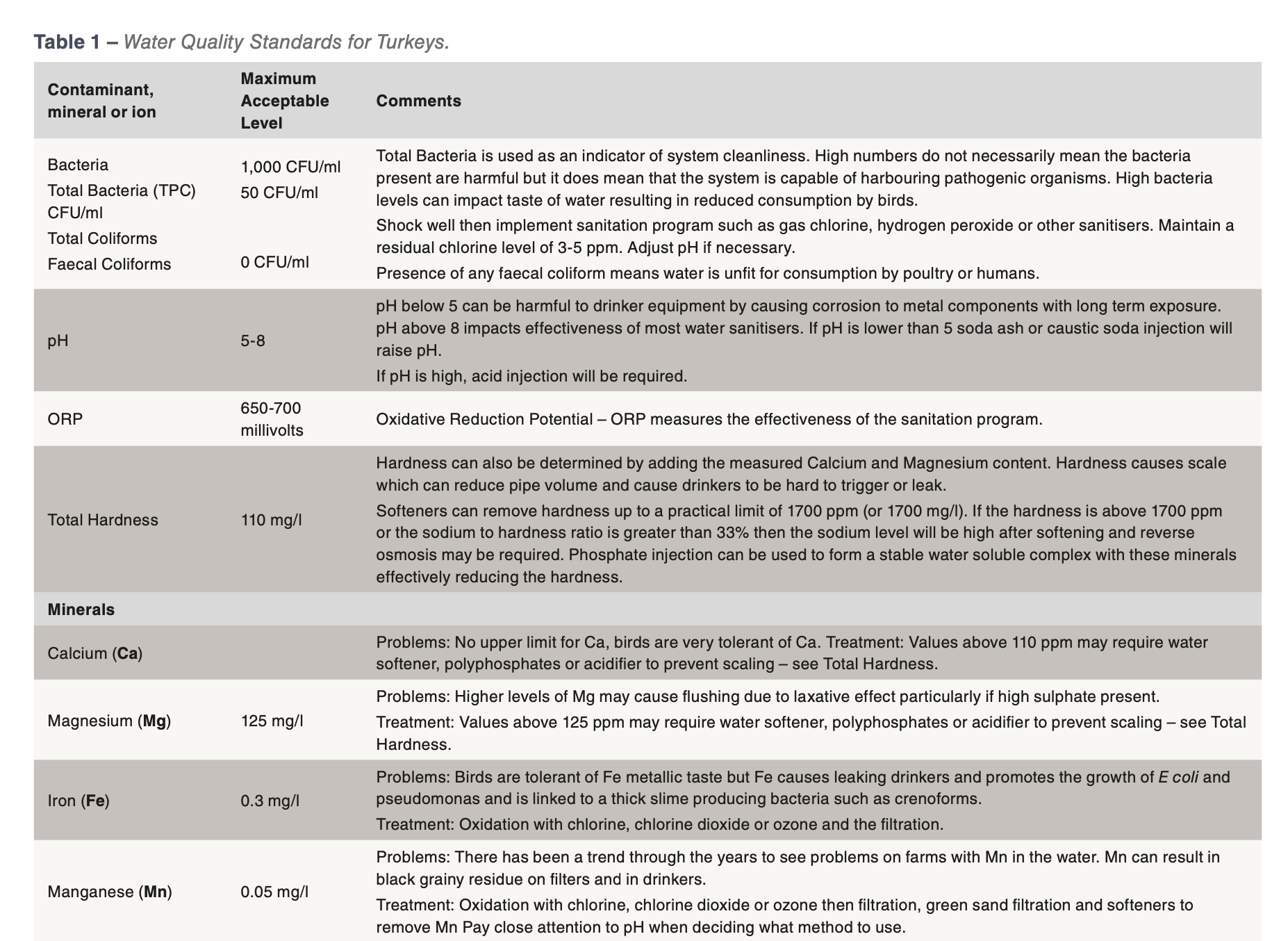
The established guidelines for microbial and mineral water quality for poultry are outlined in Table 1: Water Quality Standards for Turkeys. This table and the factors outlined below should be used to develop a daily waterline sanitation program applicable for the local conditions of the farm.
Bacteria
The microbial or bacterial test results received from labs are Total Plate Count of Aerobic (oxygen loving) Bacteria (TPC) as measured by CFU/ml (Colony Forming Units/ml). These results do not indicate whether the bacteria present is harmful or harmless but it can tell if the system is dirty and therefore at risk to the presence of less desirable bacteria.
If the total plate count or TPC level is 1,000 CFU/ml or less then the water supply is considered acceptable. On farms with excellent water sanitation it is common to see water tests which show 0 CFU/ml even from the end of the drinker line. The closer the water sample results are to 0 CFU/ml the better the water supply is for the modern turkey. Should the test results be greater than 10,000 CFU/ml, it is strongly recommended that the water system be thoroughly cleaned between flocks with an approved cleaner at appropriate concentrations and length of time and then a daily water sanitation program implemented when birds are present.
pH
pH is the measure of how many hydrogen ions are in solution and is measured on a scale of 1 to 14 with 7 being neutral. A pH reading below 7 indicates an acid with the acidity becoming greater as the numbers become closer to 1. Numbers above 7 are in the basic range of the pH scale. Making a pH change of 1 unit makes a 10 fold change in acidity or alkalinity. So a pH of 6 is 10 times more acidic than a pH of 7.
While pH is not a chemical or specific contaminant, it can impact water quality. It impacts the effectiveness of disinfectants such as chlorine. If water has a high pH then it may be necessary to acidify the water in order to create a favourable pH for effective sanitation with chlorine. Chlorine is most effective in the range pH 4 to 7, but loses effectiveness above pH 8.
Hardness
Hardness is a measure of the calcium and magnesium in the water. The biggest problem with these minerals is the scale that they form. Scale can reduce the volume of pipes and impact nipple drinkers. It also reduces the effectiveness of cleaners and disinfectants. A water softener can be used to reduce hardness. However sodium based water softeners should not be used if the water already has a high level of sodium.
Minerals
There is no such thing as pure drinking water as all sources have some amount of minerals dissolved in it. The majority of the time, these dissolved minerals are well within acceptable ranges as the turkey has been shown to be very tolerant of some minerals such as calcium and sodium but very intolerant of minerals such as iron and manganese. Iron and manganese tend to give water a bitter metallic taste and iron also supports microbial growth such as pseudomonas or E. coli. There are many cases of mineral contaminants that are not within desired levels which results in the following issues:
- Poor performance.
- Equipment failure or damage.
- Presence of harmful bacteria or fungal slime (some minerals can act as a food supply for these).
The minerals calcium and magnesium are the sources of scale, a hard white deposit found in water pipes.
If the water supply contains more than 60 ppm of either or both these minerals and the water pH is above 7 then chances are high that there is scale in the water system that will have to be removed with an acid cleaner designed for nipple drinker systems.
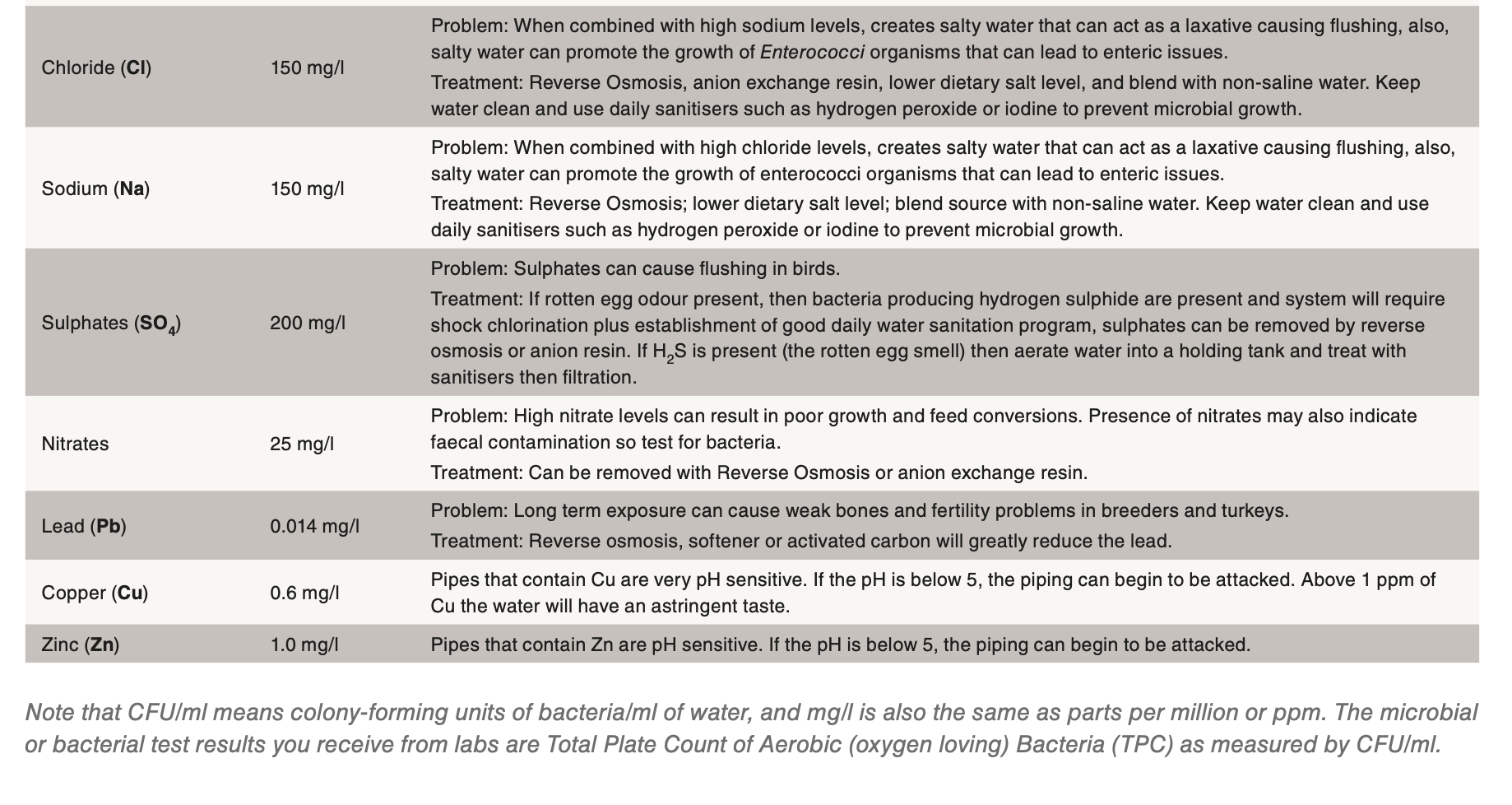
Other common mineral contaminants are iron, manganese and sulphur. Iron results in a rusty brown to red coloured residue, while manganese and sulphur can form black coloured residues. Natural sulphur in the water should have a smell similar to a match head. If the water smells like rotten eggs, then the culprit is hydrogen sulphide. Hydrogen sulphide is a by-product of sulphur loving bacteria and the lines will need to be cleaned with a strong sanitiser. It might even be necessary to shock chlorinate the well. If the filters at the beginning of the water lines are rusty or black coloured, then a strong acid cleaner should be used after the sanitiser flush. If iron is a concern, the best method of control is chlorination and filtration.
Nitrates are colourless and odourless and the only way to detect its presence is by testing. As little as 10 ppm nitrate can impact performance causing reduced growth rates and poor feed conversions.
Cleaning water lines between flocks
Providing a clean, safe and sanitised water supply is crucial in assuring flocks perform their best. Before implementing a daily water sanitation program, it is important to thoroughly clean as much of the water distribution system as possible to remove biofilm, scale and other deposits.
Daily water line sanitation
Cleaning the water lines between flocks is only half the battle. Even with a thorough cleaning, if a significant number of bacteria, fungi or yeasts are still present, then the biofilm has the potential to return completely in 2-3 days. Therefore the last step is to establish a daily water sanitation program. This will benefit both the birds and the water system. Also many of the popular water additive products such as acids and performance enhancers can create conditions favourable for the growth of yeasts and moulds, if they are present. Yeasts and moulds can actually thrive in low pH water resulting in a gooey slime that will clog drinkers and generally create disaster in water systems.
Start birds on fresh sanitized water with 3-5 PPM free chlorine residual at the end of the line or in the drinker farthest from chlorine injection. Add a second injector or medicator and inject an approved acid if the pH is too high. This will enhance the effectiveness of the chlorine.

Measuring water line sanitation
An important piece of information to know how effective the sanitisation program has been is the ORP value of the water. ORP stands for oxidation-reduction potential and it simply refers to the property of sanitisers such as chlorine to be a strong oxidiser. A strong oxidiser literally burns up viruses, bacteria and other organic material present leaving water microbiologically safe.
An ORP value in the range of 650 millivolts or greater indicates good quality water that can be effectively sanitised by as little as 2 to 4 ppm free chlorine (See Figure 1). A lower ORP value such as 250 millivolts indicates a heavy organic load that will most likely overwhelm chlorine’s ability to properly disinfect the water.
The ORP meter can be a useful tool for identifying water supplies that don’t have adequate free chlorine and for adjusting this without overusing chlorine. It is important to measure the free chlorine level in water. Water with a heavy organic load would result in a greater percentage of bound chlorine resulting in a poor sanitisation.
The bottom line is utilise information on pH, ORP and chlorine level to determine if the sanitation program is effective and to also prevent equipment damage by the overuse of chemicals.
Do not add chlorine when administering vaccines, medications, or vitamins. Do not mix chlorine and other products in the same stock solution.
















