
There is an increasing amount of published surveillance and diagnostic submissions data, providing information on prevalence and trends of antimicrobial drug use and antimicrobial resistance (AMR) in agriculture.
Enteric bacteria and antimicrobial drug resistance on poultry units in UK and Europe
The present summary draws upon periodic reports published by UK authorities [1,2] and the European Union [3,4]. In the UK in 2017, 99% of Salmonella isolations came from statutory or voluntary surveillance submissions. Around 15% of production flocks and 2% of breeding flocks were Salmonella-positive, with Derby being the most common serovar.
In recent years, Salmonella Derby has also regularly featured among the few serovars isolated from breeding flocks. In 2017 Senftenberg was the only serovar isolated from breeding flocks; this was considered to be hatchery and possibly feed-related, and also the only serovar isolated from birds originating outside the UK (France).
Antimicrobial resistance testing was reported in 2017 for both Salmonella and the related organism E. coli (the latter typically a normal inhabitant of the healthy gut) for UK turkey and broiler flock isolates. Current practice is to test and report resistances for only the first Salmonella isolate from each incident to avoid biasing national data if several isolates of the same serotype or phage type are reported from the same group of animals.
A moderate proportion (23%) of turkey Salmonella isolates were fully-susceptible to the tested panel of antimicrobial drugs, up from 8% in 2015, but much lower than the proportion of pan-susceptible isolates from chickens (79%). Few turkey or chicken isolates exhibited fluoroquinolone (FQ) resistance: 0.6% and 0.5%, respectively. By contrast, among E. coli, FQ resistance was exhibited by 12% of turkey isolates (albeit in a small sample size of 33) and less than 2% of broiler E. coli – the last down from 18% in 2015.
The extended-spectrum cephalosporin (ESC) resistance phenotype, was seen only in turkey isolates of E. coli from Northern Ireland, and not among Salmonella from either turkeys or chickens. The ‘common’ resistances seen in turkey Salmonella isolates (sulphonamides, tetracycline, streptomycin) were present at three to six times the frequency observed among Salmonella from broiler chickens.
Data from the European Union and European Economic Area countries’ National Control programmes in 2016 indicated around 6% of turkey production flocks and 2.6% of breeding flocks were Salmonella-positive. Among Salmonella isolates from turkeys and broilers, FQ resistance was overall substantially more common than in the UK (51% and 54%, respectively) and was concentrated in Eastern and southern parts of Europe. From a smaller set of 12 countries, data from turkey E. coli isolates nonetheless showed some similar patterns, with FQ (ciprofloxacin) resistance being common (46% isolates) and concentrated in eastern and southern countries. For both Salmonella and E. coli, FQ resistance was more common than quinolone (nalidixic acid) resistance, which suggests involvement of potentially transmissible plasmid-borne resistance genes. This was also reflected in Salmonella isolates from turkey meat.
Resistances to commonly-used antimicrobial drugs (sulphonamides, tetracycline, ampicillin) were frequent among turkey Salmonella and E. coli isolates. Similarly, resistance to three or more antimicrobial classes (multi-drug resistance; MDR), was evident in 43% and 49% of Salmonella and E. coli isolates, respectively. By contrast, the ESC phenotype was seen in less than 1% of Salmonella and 2.7% of turkey E. coli isolates overall.
More frequent ESC-type resistance was seen among turkey E. coli in Southern and Eastern Europe, and in Spain nearly 4% of Salmonella isolates were similarly resistant. Broiler isolates infrequently exhibited the ESC resistance phenotype (generally <2% of Salmonella and <4% of E. coli isolates by country), although 12% of Italian Salmonella isolates were ESC-resistant. The use of selective media to screen caecal contents at slaughter yielded evidence of more widespread ESC E. coli (36.6% of samples) in a prospective study in 12 countries.
Campylobacter monitoring in turkeys was reported in Europe only by Iceland in 2017, which isolated the organism from 2.8% of fattening turkey batches. Eleven countries reported Campylobacter isolations from turkey meat, at an overall prevalence of 32% of samples compared with 37% of broiler meat samples. Of 1060 Campylobacter jejuni isolates from birds and meat from 9 member states, the prevalence of FQ (ciprofloxacin) resistance was high overall (76%) but varied widely by country (<10% to >90%). MDR was low, at 1% of isolates.
Antimicrobial drug use – UK
The following is summarised from the UK VARSS report [2], covering 90% of UK poultry meat production.
Antimicrobial drug use by the turkey industry in 2017 was reduced by 48% and 79% over 2016 and 2014 figures, respectively, using a standardised measure of milligrams per kilogram bodyweight at time of treatment (mg/kg). The intensity of use for turkeys in 2017 (45.2 mg/kg) is similar to that in the broiler sector in 2014, which latterly has achieved usage below 10 mg/kg.
For turkeys, much of the reduction in recent years has been in use of tetracycline: from 165 mg/kg (75% of total antibiotic use) in 2014, to 22 mg/kg in 2017. The reduction in fluoroquinolone use has been even more marked: a drop from 7.4 to 0.4 mg/kg between 2014 and 2017, representing a reduction of 95%. Extended-spectrum cephalosporin antimicrobials have not been used in the turkey sector since 2012.
Risk factor studies for antimicrobial resistance on poultry units
There are few formal published risk factor studies that address relationships between farm factors and the presence or degree of AMR on poultry units. A multivariable analysis of E. coli in UK turkey production [5] found the presence of cephalosporin resistance in fattening flocks to be associated with certain external biosecurity factors (nearby pig units, staff working with other livestock), whilst some flock separation measures (dedicated gloves and house partitions) appeared protective (P≤0.09). The same study also considered FQ (ciprofloxacin) resistance. For turkey fattening flocks, any antimicrobial use in the flock or evidence of mouse activity was weakly associated with an increased risk of resistant E. coli being present (P≤0.1), whilst routine disinfection at depopulation and partitioning of houses appeared protective.
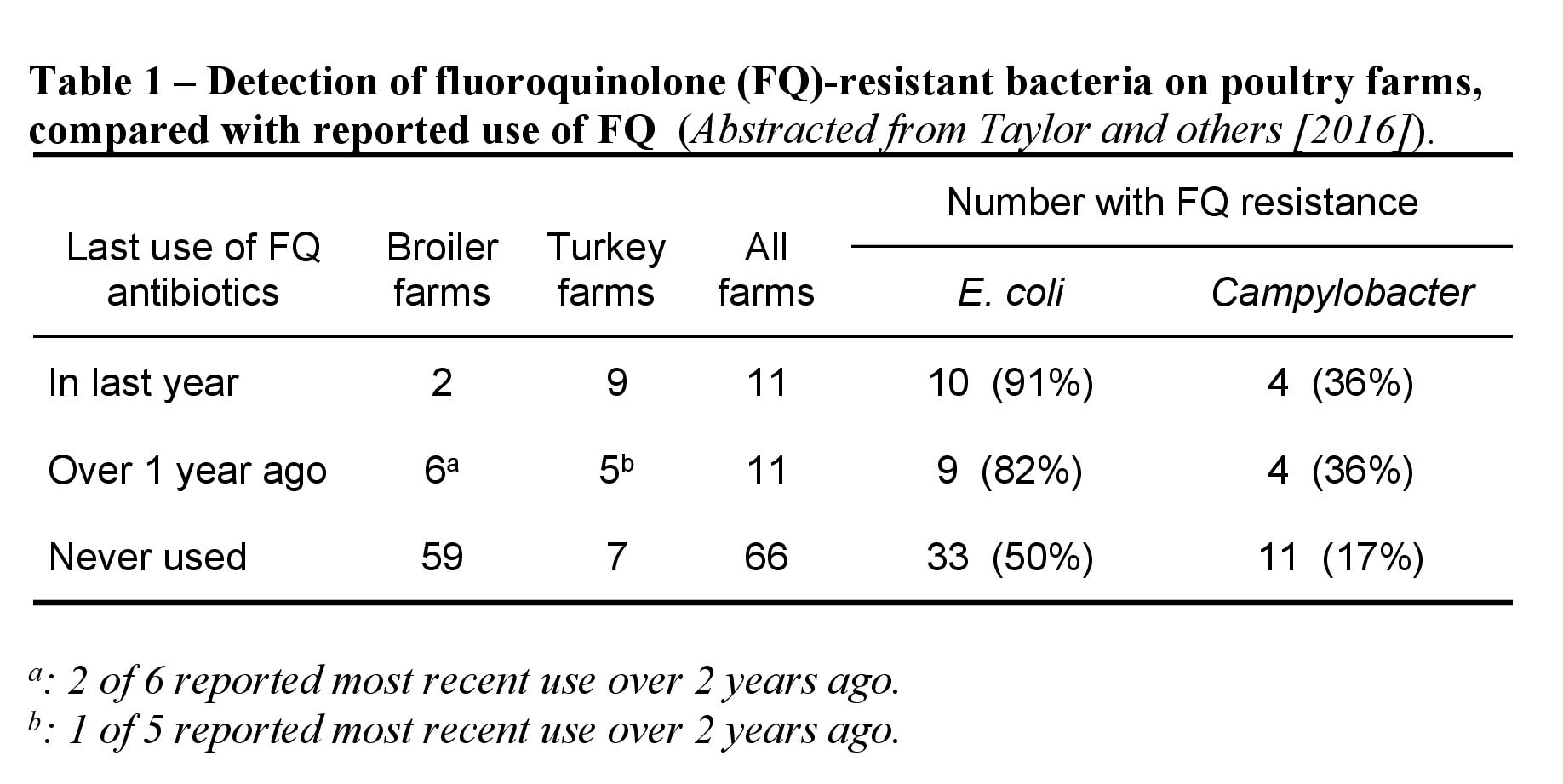
Taylor et al. [6,7] examined pooled droppings from 21 turkey and 67 broiler farms, comprised of a mix of integrated and independent growers sampled around 2002. FQ (ciprofloxacin)-resistant E. coli and thermophilic Campylobacter were isolated on selective media. No use of FQ was reported on 33% of turkey farms and 88% of broiler farms. Nonetheless, FQ resistance among E. coli was found on 90.5% of turkey units and 50% of broiler units; equivalent figures for resistant Campylobacter were 38.1% and 17.6% of turkey and broiler units, respectively.
Turkey and broiler farms were considered together in multivariable models relating representative candidate factors and farm FQ-resistance status for each organism. FQ use was clearly a significant risk factor for both FQ-resistant Campylobacter and E. coli, but resistance was present on several units without reported use of such antimicrobials (Table 1). Of the additional factors for Campylobacter at or near conventional significance, wild bird access to poultry houses was a positive risk (Odds Ratio[OR] 10.3, P<0.017) whilst three hygiene measures (provision of a face mask to staff, all detailed areas being dusted, and feed hoppers being cleaned and disinfected) were apparently protective. These negative risk factors were variously and positively correlated with other hygiene practices: wet cleaning of all detailed areas with disposal of wash water off-site, C&D of ante rooms, provision of hat and gloves, hand sanitizer and a toilet. Larger and independently run farms were also at lower risk.
For the presence of resistant E. coli, certain biosecurity features appeared to be risk factors, i.e., public perimeter path (positive; OR 4.1, P=0.019) and perimeter fence (protective; OR 0.27, P=0.014). Single-handed operation was also a positive risk factor.
In Norwegian commercial broiler production, and in the absence of antimicrobial use, a multivariable model revealed associations between the presence in a flock of cephalosporin-resistant E. coli (AmpC-type) and: the previous flock being similarly positive (OR 12.7, P<0.001), more parent flocks contributing to the production flock (3 versus 1 parent flock: OR 6.3, p=0.01), chick delivery personnel being allowed access to the broiler house (OR 9.3, P=0.01), and consistent floor disinfection between flocks (negative association; OR 0.1, P=0.01) [8]. A Canadian univariable study [9] observed that rearing of turkeys on litter previously used by a broiler chicken flock was associated (P=0.04) with carriage of ceftiofur-resistant E. coli in the turkeys at slaughter.
Discussion
The presence of zoonotic enteric organisms (such as Salmonella and Campylobacter) in flocks of turkeys and other poultry is rightly a matter of concern for the health and welfare of consumers and farm workers. There is also increasing attention being paid to the incidence of AMR among such bacteria, and even among commensal organisms (such as E. coli) that may harbour mobile resistance elements, such as plasmids, that can transfer AMR to other bacterial species. The environment of the gut is likely to be one where such transmission of resistance is facilitated.
There is a clear and highly-plausible link between the use of antimicrobial drugs in poultry production and the presence, or degree, of antimicrobial resistant organisms in flocks. This has driven recent policy and initiatives in respect of antimicrobial use in flock husbandry and medicine, and in the UK there has, in consequence, been a remarkable reduction in the use of such drugs for poultry in recent years.
Turkey production continues to use substantially more antimicrobials than broiler chickens, and the patterns of common resistances and of fully-susceptible Salmonella isolates in the two sectors appears to reflect that, although in both sectors there is an improving trend in recent years. Resistance to critically-important drugs (FQ and ESC) is, happily, low among UK poultry Salmonella isolates. By contrast, the prevalence of ESC resistance in Europe is a little higher, and FQ resistance is much higher.
A few individual countries report a substantially higher prevalence of a certain resistance in a certain sector. This is true also of FQ resistance in Campylobacter isolates from broilers and turkeys, including in the UK despite minimal FQ use in recent years. This may be related to enhanced biological fitness and a resulting ability of some FQ-resistant strains to circulate within and between poultry companies [10,11]. By contrast, macrolide resistance carries a fitness cost and remains uncommon amongst poultry Campylobacter strains in most countries [12]. Where risk factor studies for the occurrence of AMR on poultry farms have been done, and particularly in the case where antimicrobial drugs were not used in the studied units, it is evident that other factors in addition to the use of antimicrobial drugs influence the patterns of resistance seen.
Risk factor analyses need careful interpretation, as they can yield implausible statistical associations, findings can point to proxies rather than linked causal factors, or initial data gathering may miss potentially important factors. However, a strong and highly-plausible theme emerging from the few studies that have been performed in this field, is that biosecurity and hygiene measures, when present or well-performed, are associated with a lower risk of AMR in target organisms (E. coli and Campylobacter have been studied) on premises or in flocks. This is in addition to effects of antimicrobial drug use, although it may reasonably be considered that there is an additional linkage between poorer biosecurity and the frequency of antimicrobial drug use [13].
Some of the causal relationships behind these observed risk factor correlations are illuminated by tracing studies, whereby ESC resistances in broiler E. coli isolates can be related to imported breeding stock [14] or to certain hatcheries [15].
The prevalence data from Europe shows regions and pockets of elevated AMR to critical antimicrobials among enteric bacteria, and illustrates the consequent risk of importing such organisms and their transmissible resistance elements into areas (such as the UK) where such resistance is generally infrequent. Thus, there is emerging evidence that minimising or eliminating AMR requires high standards of biosecurity and hygiene at all stages of the production chain, from breeding flocks through hatcheries to fattening units, in addition to minimising the amount and types of antimicrobial drugs used.
References are available on request
From the Proceedings of the 13th Turkey Science and Production Conference

















