
When vaccination against infectious laryngotracheitis (ILT) is necessary in broiler flocks, mass administration techniques are required due to the sheer numbers of birds involved. This is usually done by drinking water administration even though ILT vaccines are generally registered for individual eye drop application.
Often the results are variable, with occasional outbreaks occurring in vaccinated flocks or rolling vaccine “reactions” occurring, sometimes involving clinical signs and even mortality. A field evaluation study was performed during a field outbreak using collection of tracheal swabs from birds in vaccinated sheds. DNA extracted from swab samples was submitted for quantitative real time PCR (qPCR) to detect and quantify ILT virus (ILTV) at the University of New England (UNE).
The first shed examined used 70 individually identified birds which were retrieved at 7 post vaccination time points. A further 7 sheds were then followed with 40-45 randomly selected birds at 4, 7-8, 12-13 and 25-26 days post vaccination. Vaccination procedures and timings of administration were recorded. Patterns of vaccine “take” were compared across factors recorded at vaccination day to look for putative risk factors for poor or better vaccination success. ILT virus recovery varied markedly between sheds but there were patterns observed between poor and somewhat better results.
Factors showing statistical association with these patterns included use of a dye product to stabilize water as a risk factor and longer time of water stabilization with a skim milk product prior to vaccine addition as a protective factor. There was also an almost significant (P=0.07) association of chick source with vaccine take pattern. Results have implications for vaccine application and suggest a possible monitoring process for vaccination success.
Introduction
Field success of mass ILT vaccination in young meat chickens is often accompanied by reports of vaccine reactions, often rolling through the flock for some time, and even apparent vaccination failure with wild strain outbreaks in vaccinated flocks. This is in spite of laboratory challenge studies often describing good protection of vaccines against an artificial challenge with the field outbreak strain. Laboratory studies have described post vaccination (pv) recoveries of vaccine virus in directly vaccinated birds and in-contact birds.
Using the Serva ILT vaccine strain (MSD Nobilis; Class 7 virus), these studies have shown that directly vaccinated birds (eye drop route) have a peak tracheal viral copy number at day 4 pv, while birds placed in contact at day of vaccination show a delayed peak at day 8 pv. Birds vaccinated by drinking water in these laboratories however show a peak tracheal ILT viral titre at day 8 pv, which would tend to indicate that there are some birds receiving contact with the virus by spread from other birds.
Further, some studies showed that birds placed in contact with Serva vaccinated birds at various times showed intermittent ILT viral recovery from trachea for extended periods with intermittent levels of mucosal antibody in the trachea cited as being responsible for this pattern.
In 1981 it was demonstrated that ILT vaccine virus must make contact with a respiratory target tissue (e.g. conjunctiva, nasal passages, and trachea) to establish an infection and replicate and thus stimulate immunity but contact only with the mouth, tongue, pharynx or oesophagus was ineffective.
It was also showed that drinking water application only achieved this respiratory contact by accident, with less than 30% of birds showing such contact, and only with a limited amount of the vaccine administered. In 2013, de Wit further notes that for ILT vaccination via drinking water to be as effective as eye drop, multiple doses (10 to 100 times) of the vaccine are required.
To evaluate some aspects of what actually occurs during a field mass vaccination application of ILT vaccine in broiler chickens, a study was performed to look at sequential ILT vaccine virus detection in tracheas in several flocks.
Method
Study 1. A natural ILT outbreak induced by a wild ILT strain classified as Class 9 began in the greater Sydney region in mid-2017. The local poultry companies began vaccinating broilers in this region with Class 7 (Serva) strain vaccine. A single shed on a 5-shed farm that was being vaccinated was selected at random for the initial study. Vaccination was administered in drinking water using skim milk powder as a stabilizer at 9 days of age. Seventy chicks were selected at random from various locations in the shed 1 day pv. These had individual tracheal swabs collected and they were individually identified with coded permanent pen markings on each shank and their wings were sprayed with blue stockmark to enable them to be located at the next sampling day. The birds were retrieved on each of days 4, 8, 12, 18, 21 and 26 pv when tracheal swabs were again collected and the identification markings refreshed. Apart from one bird which died from a non-ILT cause on day 15 pv, every bird was retrieved at every sampling occasion. DNA was extracted from swabs at Birling Avian Laboratories (BAL) and samples submitted to UNE for ILT qPCR.
Study 2 – Based on the findings of study 1, a sample size of 40 was calculated as statistically able to provide a valid estimate of prevalence of ILT detectability from tracheas and another 7 sheds were selected across three farms. Forty or 45 birds were randomly selected at each of days 4, 7-8, 12-13 or 25 pv and tracheal swabs were collected from each and again submitted for qPCR to enumerate ILTV genome copy number. In all sheds, vaccination procedures were documented, including times between critical procedures during administration and choice of water stabilizer used. Two sheds on the final farm agreed to use a dye preparation as stabilizer as a comparison to a skim milk product (either powder or liquid).
Point and period prevalence of ILT viral detection in tracheas was calculated at each sampling time. Statistical analyses were conducted using STATISTICA v6 software comparing prevalence across vaccination administration factors.
Results and discussion
In study 1 with repeat sampling of the same birds, the incidence of birds (i.e. those first showing a positive result on each day/number remaining negative at the previous day) with positive tracheal detection of ILT virus was only 2 birds positive at day 4pv (2.85%), 4 at day 8 pv (6.06%), 13 birds at day 12 pv (20.3%), 13 birds at day 18 pv (25.4%), 10 birds at day 21 pv (27.0%) and 18 at day 25 pv (66.6%).
Period prevalence (i.e. the total number of birds with ILT virus detectable from tracheal swabs on each sampling day) was 2.9%, 8.6%, 27.1%, 46.4%, 60.9% and 87.0% at each time point respectively. Thus, from a very few number of birds positive at the first sampling, the numbers of birds showing detectable virus grew slowly over time.
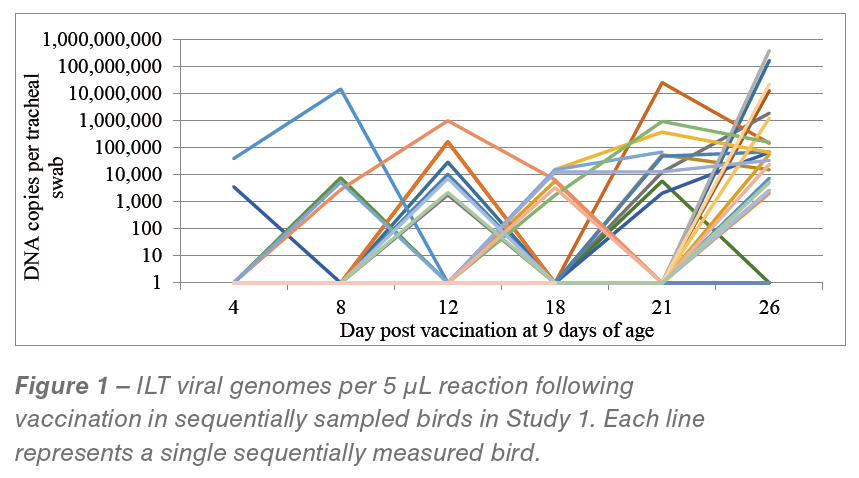
There were still 9 birds (13%) in the sampling group which never showed ILT viral detection up to day 26 pv (or 34 days of age when depopulation of the flock began). Also many birds revealed an intermittent presence of ILT virus in their tracheas over time. Quantification of viral DNA in tracheas over time for this study is shown in Figure 1.
This intermittent pattern is very evident and it can be seen that viral loads did not peak at 8 days, as would have been expected from laboratory studies using drinking water administration of Serva strain vaccine.
It was obvious that bird to bird transmission was mostly responsible for the eventual vaccine spread through this flock. All positive isolates on day 25 pv were typed at BAL and all proved to be Class 7, consistent with the vaccine strain. No Class 9 strain was detected in this flock. It was concluded that the uptake of vaccine virus from the actual drinking water application in this flock was very poor.
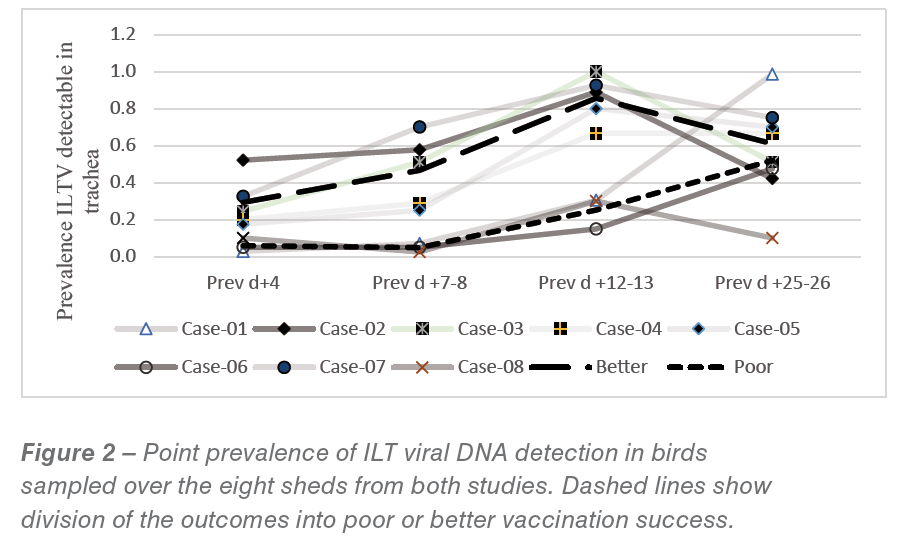
Period prevalence from study 2 (and including results from study 1) for detection of ILT viral DNA from tracheal swabs is shown in Figure 2. Two obviously different patterns of apparent success of vaccination could be discerned from Figure 2, with 3 flocks showing a “poor” uptake and 5 flocks showing an earlier “better” uptake. None of the flocks could really be regarded as having a “good” vaccine uptake from the drinking water administration (the prevalence of tracheal ILT DNA at day 4 pv varied from 2.8 to only 52%). The flocks in the “better” category had significant proportions of the flock exposed to the vaccine virus by 12-13 days pv (i.e. around 21-23 days of age).
Most field outbreaks are reported after 30 days of age and hence, given an incubation time of 6-8 days (AV Dis ref), spreading wild virus infection may be occurring around 22-24 days. Flocks with high vaccine prevalence after 12-13 days pv therefore may have significant protection but those in the “poor” category would still have large numbers of susceptible birds at this age.
This may explain the field observation of vaccine failures. Also, bird to bird transfer of vaccine strains can lead to reversion to virulence, and the slower spreading of vaccine virus in the “poor” take flocks may be responsible for the observed vaccine “reactions” in the field.
Contingency table analysis across the two vaccine “take” patterns showed significant associations of the “poor” takes with the use of the dye stabilizer product as compared to skim milk products and with shorter stabilization time before vaccine was added. There was also a possible association with hatchery source of the chicks (P=0.07) with prevalence of tracheal ILT DNA detection at 12-13 days, but this needs to be further explored.
Drinking water administration of ILT vaccine continues to be a gamble. Uptake of the vaccine virus from this mass vaccination route appears to be fraught with inconsistency and studies aimed at understanding which techniques result in better vaccine uptake need to be expanded in order to improve vaccinal protection for susceptible broilers in the face of outbreaks. This was a restricted study in terms of sheds studied so wider observational studies aimed at confirming the putative risk factors identified here are necessary to validate the conclusions on risk factors.
References are available on request
From the 2019 Australian Poultry Science Symposium

















