The broiler chicken meat processing industry produces a large amount of poultry byproducts rich in connective tissue (i.e. keel cartilage, skin, and bone residues), which can be exploited for isolating important natural health ingredients. Connective tissue contains the extracellular matrix that includes collagen, glycoproteins, proteoglycans and glycosaminoglycans (GAGs).
Proteoglycans are the major components of this extracellular matrix and are comprised of two types of molecules: the glycosylated protein core and covalently attached sulfated GAGs. These acid polysaccharides from various animal sources have been extensively studied. They are described based on their disaccharide composition and degree of sulfation; on one hand, the sulfated GAGs, such as chondroitin sulfate (CS), dermatan sulfate (DS), keratin sulfate (KS) and heparan sulfate (HS) are all bound glycans to the protein core; and on the other hand, hyaluronic acid (HA), the non-sulfated GAG polysaccharides, all exist as a free polymer.
Sulfated GAG polysaccharides can be liberated from the extracellular matrix by enzymatic or chemical hydrolysis and are considered biologically active compounds. They have a wide range of applications in pharmaceutical and food industries. For instance, CS is used as a supplement for treating osteoarthritis due to its anti-inflammatory and chondro-protective effects.
HS and DS also have anticoagulant activities. Besides these known properties, recent in vitro studies suggest that GAG polysaccharides can enhance non-heme iron absorption, thereby improving one’s nutritional iron status. For instance, the GAG-containing fraction of cooked haddock has increased the iron uptake of epithelial Caco-2 cells in a simulated gastrointestinal model. Hence, this opens the possibility of extracting GAGs from poultry processing by-products with the primary aim to produce bioactive compounds that can be used in product formulation to produce functional foods.
Extraction and separation of GAGs
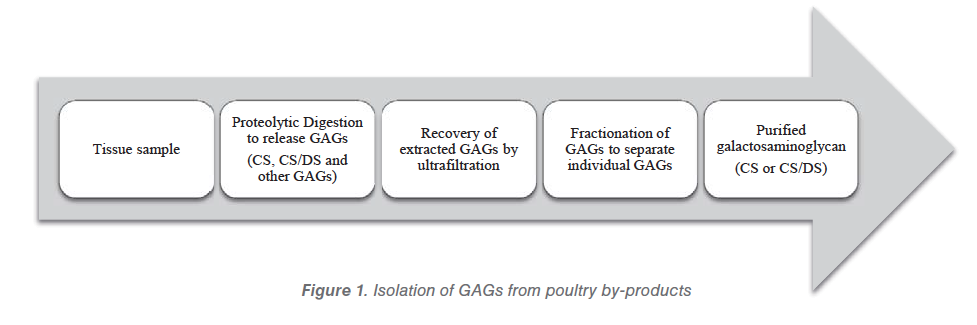
Different selective extraction procedures and precipitation techniques have been used to isolate and obtain different types of sulphated GAG polysaccharides. Various attempts have been made to minimize the use of organic toxic solvents and chemicals during the extraction and separation processes. A food grade extraction according to the methodology of Nakano et al. (2012) was conducted on pre-cleaned chicken skin and cartilage tissues. This extraction involves the use of an economical proteinase for liberating the sulphated GAG polysaccharides from the extracellular matrix and a subsequent membrane ultrafiltration at 10 kDa molecular weight cut off (MWCO) to replace the common hazardous chemical deproteinization step involving trichloroacetic acid. The extraction of food grade GAGs from poultry byproducts is reported in the Figure 1.
Tissues
Cartilaginous tissues are the major source of CS, while most galactosaminoglycans in non- cartilaginous connective tissues (e.g. skin, tendon and skeletal muscle epimysium) are CS/DS. Tissue samples are cut into small pieces. Cartilage tissues with relatively low fat content may be used for GAG extraction without defatting.
Bones are crushed with a hammer, frozen in liquid nitrogen and ground while still frozen in a Wiley mill to obtain a small tissue size. Bone tissues need to be decalcified using EDTA or acid (e.g. HCl). Without decalcification, efficient proteolysis of bone tissues is difficult to achieve.
Proteolysis
Whole GAGs can directly be liberated from tissues by hydrolysis with exogenous enzymes (i.e. pancreatin). GAGs can also be liberated from cartilage by activation of endogenous enzymes (autolysis) without using exogenous proteinase.
Recovery and fractionation
The GAGs liberated by proteolysis are the subjected to an ultrafiltration step with 10 kDa MWCO. GAGs have a MW of around 20 kDa, therefore they can be easily separated from the peptides (mostly collagen peptides). After ultrafiltration, ion exchange chromatography using a gradient of NaCl is used to fractionate the GAGs.
Two mains fraction are obtained: the 0.4 M, containing mostly unsulfated GAGs and the 2.0 M NaCl which contains the sulphated GAGs (i.e. CS and DS). The 2.0 M fraction is then subjected for selective precipitation by using different concentration of food grade ethanol in order to recover different types of sulphated GAGs.
Bioactivity of GAGs
GAGs have been used clinically for the treatment of chronic diseases such as degenerative arthritis, cirrhosis and chronic photo damage due to the emerging bioactivities such as antioxidant, anti-atherogenesis, anticoagulation, prevention and cure of arthritis. Their bioactivities depend on their specific chemical structure, including the patterns and degree of sulfation, molecular mass, relative amounts of iduronic (IdoA), glucuronic acids (GlcA) and hexosamine. For instance, HS is used as an anti-coagulant, while HA as a component in the synovial fluid lubricant in body joints. CS is used in the treatment of cartilage and tendons. Despite these known applications of GAGs, novel function and uses are emerging.
Of interest is the possibility of using low molecular weight GAGs (i.e. oligosaccharides) as a prebiotic or for their ability to increase micronutrient bioavailability.
GAGs and iron bioavailability
Fe deficiency is a common and debilitating primary nutritional disorder, and can result in anaemia and brain development abnormalities. Fe deficiency is still common in the developed world and keeps many people from thriving. The promotion of Fe absorption is a practical way of dealing with modern micronutrient deficiencies. Certain components in muscle tissue can enhance Fe absorption, a phenomenon called the “meat factor”.
Active molecules that enhance Fe bioavailability have been isolated from fish and meat processing by-products, like fish skin collagen. Recently, Wang and Betti (2017) demonstrated that GAGs extracted from poultry skin and cartilage possesses a certain antioxidant activity and ability to increase iron bioavailability in intestinal cell culture model.
Ferritin formation in intestinal culture model was also enhanced by the hydrolysis treatment, indicating that the GAGs-derived oligosaccharides have ability to increase the iron bioavailability.
These results are of practical importance and may represent a very significant way to add value and increase sustainability of the meat processing industry and its bproducts. For instance, current studies in my research group are focusing on the supplementation of GAGs in several food matrix including milk products. The aim is to create food product that have ability to increase the bioavailability of key-micronutrient like iron.
References are available on request
From Proceedings of Midwest Poultry Federation Convention