
Conventional broilers often experience intestinal problems between 20 and 30 days of age, caused by a microbial imbalance in the gut, which is referred to as dysbacteriosis and is associated with wet litter and decreased feed intake resulting in poorer feed conversion and reduced body weight. The diagnosis of dysbacteriosis is based on macroscopical scoring of intestinal damage. At necropsy, dysbacteriosis is generally characterized by intestinal inflammation, thinning of the gut wall along with foamy or watery intestinal content.
Although the exact pathogenesis is still unclear, dietary stressors such as soluble non-starch polysaccharides and enteric infections caused by Eimeria spp or bacterial/viral pathogens are factors possibly involved in the onset of dysbacteriosis. It is a challenge to develop a model that mimics dysbacteriosis and thus yields high macroscopic lesion scores, microscopic mucosal defects and performance decreases.
How we investigated or researched the problem
In the present study Authors evaluated different experimental models to reproduce dysbacteriosis. Therefore, day old male chicks were randomly allocated to 4 groups (Table 1).
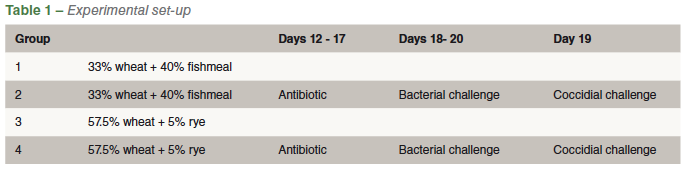
The chickens of groups 1 and 2 were given a wheat (33%) based diet supplemented with 40% fishmeal, which is reported to induce significant changes in the gut microbiota. Chickens from groups 3 and 4 were fed a wheat (57.5%) based diet supplemented with 5% rye that evokes mucosal damage and increased leakage of the intestinal barrier. The animals of group 2 and 4 were administered 10mg enrofloxacin and 10mg florfenicol per kg body weight from day 12 till day 17 to induce changes in the gut microbial community.
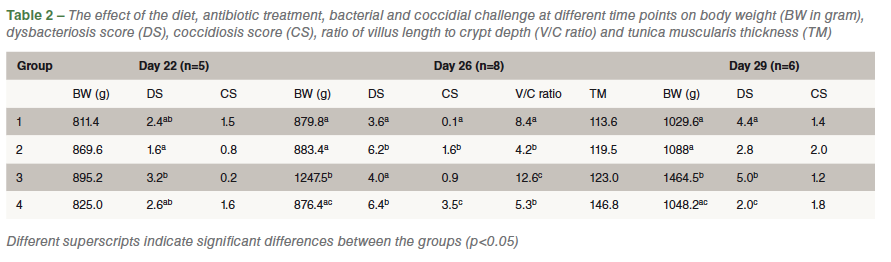
After the antibiotic treatment, a bacterial cocktail, consisting of Clostridium perfringens, Escherichia coli, Enterococcus sp, Lactobacillus salivarius and Lactobacillus crispatus, was given daily from day 18 till day 20 and a coccidial challenge consisting of 20.000 oocysts of E. acervulina and E. maxima was administered once at day 19. On days 22, 26 and 29, a number of animals per group were weighed, euthanized and macroscopically scored for dysbacteriosis and coccidiosis. On day 26, also a part of the duodenum was fixed in formalin to measure villus length, crypt depth and thickness of the tunica muscularis.
Conclusions
Challenging birds by a protocol with antibiotic treatment, followed by bacterial and coccidial challenge affected performance, macroscopic dysbacteriosis score and gut wall morphology at day 26, which is representative for dysbacteriosis in the field.
The influence of the antibiotic treatment, bacterial and coccidial challenge on the body weight is significant when the chickens are administered a high NSP containing diet (wheat + rye supplemented feed).
There is a strong negative correlation between the macroscopic scoring system and the ratio of villus length to crypt depth, suggesting that the macroscopic scoring system is a reliable tool to diagnose gut health disorders.
The developed model can be used to evaluate the efficacy of different measures to control impaired gut health in broilers.
References are available on request
From the Proceedings of the 4th International IHSIG Symposium on Poultry Gut Health

















