Infectious bronchitis virus (IBV) is an endemic disease of chickens responsible for considerable economic losses to the poultry industry worldwide. IBV replication leads to considerable viral genetic variability, and vaccine failures are commonly seen in the field. For this reason, new strategies to prevent infection and induce disease resistance must be investigated.
Genetic resistance or susceptibility to infectious diseases have been largely associated with the avian major histocompatibility complex (MHC) genes.
UC Davis has congenic chicken lines that share the same genetic background and vary solely in their MHC haplotype. Authors challenged congenic chicken lines available at UC Davis with an IBV Massachusetts 41 (M41) strain to determine which lines are relatively resistant or susceptible to IBV infection. This was accomplished by comparing clinical signs, pathology and humoral responses after challenge.
The objective was to determine the relative resistance and susceptibility of the chicken lines to IBV and establish a model to understand immunity against IBV and the relationship between the MHC and innate immune responses. The Authors analyzed and compared immunological responses and the effect of challenge in different congenic and inbred chicken lines.
Materials and methods
Five MHC B haplotype congenic lines (253/B18, 312/B24, 331/B2, 335/B19 and 336/BQ), one inbred line (003/B17), and one commercial line of broilers were used in this experiment. Twenty-five day-old chicks of each line were raised in isolated rooms, totalizing 175 animals. Sera was collected at 21 days of age to detect maternal antibodies against IBV by ELISA. At 23 days of age, all birds were challenged with a M41 strain of IBV via oculonasal route using a median embryo infective dose (EID50) of 5×107 in a final volume of 200 µL.
At two and six days post- infection (DPI), tears were collected from all chickens for viral load assessment by RT-qPCR. Respiratory signs were assessed and indexes were calculated based on the severity of respiratory disease. Five birds per group were euthanized at each time point, and tracheas were collected for histopathology and histomorphometry (tracheal epithelial thickness measurement). At 14 DPI, tears and sera were collected from all remaining birds for IgA and IgG measurement by ELISA.
Clinical signs, viral load, histomorphometry measurements, and antibody levels were analyzed individually and compared by one-way ANOVA followed by Tukey multiple comparisons test using GraphPad Prism software (GraphPad, La Jolla CA, USA). Statistical differences were considered at a significance level of P<0.05.
Results
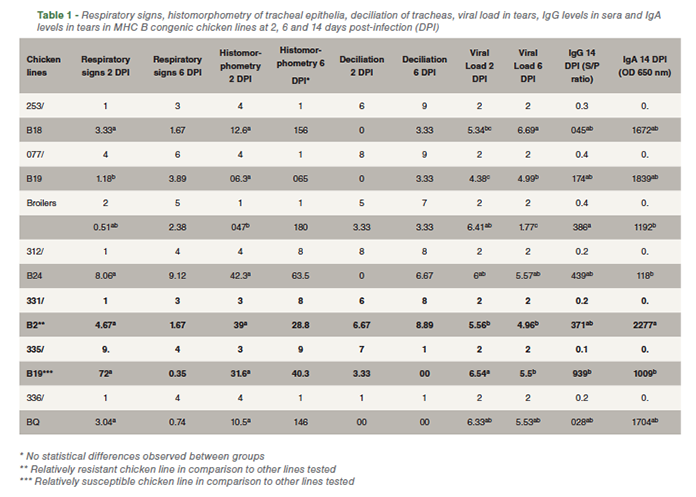
All chickens presented respiratory signs after challenge. However, mortality was not observed after infection. At two DPI, the MHC B congenic chicken line 335/B19 showed the lowest respiratory sign index and the milder tracheal inflammation. On the contrary, clinical signs were more severe in 077/B19 inbred chicken line. There were no statistical differences among groups regarding respiratory signs and histomorphometry measurements at six DPI. Lines 077/B19 and 253/B18 showed the lowest viral load at two DPI while broilers and 331/B2 showed the lowest viral load at six DPI, suggesting reduced viral shedding and consequently decreased viral replication due to early viral neutralization. Broilers presented the highest levels of serum IgG. IgA in tears was significantly higher in 331/B2 in comparison to the other tested groups. All results are summarized in Table 1.
Discussion
Even though lines 335/B19 and 077/B19 present the same MHC haplotype, the results obtained in both lines were divergent. The discrepancy is probably due to the fact that these lines do not share the same genetic background, thus other loci might be playing a role in genetic resistance against IBV in line 077/B19. Although there were no statistical differences between groups regarding clinical signs and tracheal inflammation at six DPI, there is a trend suggesting that line 331/B2 is more resistant than the others at 6 days after challenge. This trend can also be observed when considering the high levels of IgA detected in chickens from line 331/B2. As IBV preferred site of replication is locally at the upper respiratory tract, IgA levels are more likely related to protection against IBV than systemic IgG titers.
Relative resistance and susceptibility findings match what has been observed in previous experiments from the group and what has been described in literature, in which B2 and B19 present resistant and susceptible characteristics respectively.
Susceptibility differences were observed early in infection, and are most likely related with differences in innate immune function. Authors’ prospective studies will use the most resistant (331/B2) and the most susceptible (335/B19) congenic chicken lines as a model for understanding the mechanisms in which the innate immune system generates resistance to IBV in early infections. The goal is to use molecular tools and functional assays to unveil how the chicken lines respond to different strains of IBV and what are the cytokines and molecules involved in protection against IBV infection.
References are available on request
From the Proceedings of the 66th Western Poultry Disease Conference 2017

















