
This study was conducted to evaluate the effect of two different fibre sources on pullet growth, gut immune tissue, and lymphocyte proliferation of strain pullets fed three different diets from 10-18 weeks of age.
The European Union banned the use of antibiotics as non-therapeutic growth promoters for poultry in 2006 and in other countries, including Australia, there have been restrictions placed on their use. As a result, there has been increased interest in finding alternative growth promoters and immunomodulators. Finding new alternatives to antibiotics aimed at improvement of the immunity and productivity of poultry is important, especially for economic and sustainable production. As an effective alternative to antibiotics, different types of fibre have been suggested for use in the diet of layer pullets. Various types of dietary fibre have been shown to enhance body weight gain, growth of lymphoid tissue and lymphocyte proliferation of broilers and ducks. It has also been reported that a commercial insoluble fibre (Arbocel® RC fine, JRS Co. Inc., Rosenberg, Germany) and a commercial mixed soluble and insoluble fibre (OpticellC5, Agromed Austria GmbH) added to the diet of four-week old layer strain pullets for four weeks can enhance innate immune function.
The aim of this study was to investigate whether supplementing the diet of 10 to18 week old layer-strain pullets with either an insoluble (IF) or a mixed soluble and insoluble fibre (MF) product would improve their body growth, gut lymphoid organ growth and lymphocyte proliferation.
Materials and methods
Fifty-four 10-week-old grower pullets were weighed and randomly placed, three per pen, in slatted floor pens (1.8 x 0.9 m, length x width), six pens per treatment. The three dietary treatments were Control, a commercial grower pullet feed (Pullet Grower, Barastoc – Ridley Agriproducts) with no additive; Group IF (insoluble fibre), given the control diet with 1.5g/100g of a commercial lignocellulose supplement containing 65 – 70% crude fibre high in cellulose and 20% lignin (Arbocel® RC fine). Group MF (mixed fibre), given the control diet with 1.5g/100g of a commercial lignocellulose supplement containing 85% soluble and insoluble fibres, 30% lignin (OpticellC5).
After seven weeks on the diets, blood samples were taken from the brachial vein of eight pullets per treatment, 1-2 pullets/pen for isolation of lymphocytes using a modification of the method of Lavoie and Grasman. After separation of lymphocytes using Histopaque® 1077 (Sigma-Aldrich) the number of viable cells was determined by staining with trypan blue.
The lymphocytes were then plated into a sterile, clear, flat bottom 96-well plate (Falcon™, Becton Dickinson Labware). Mitogen induced proliferation of T- and B- lymphocytes was stimulated by adding either Concanavalin A (Con A, 2.5 µg/ml, Sigma-Aldrich) or lectin from lipopolysaccharide (LPS, 3.125 μg/ml, Sigma-Aldrich) to the incubation medium. Proliferation of lymphocytes unstimulated by mitogens acted as a proliferation control. After 2.5 days incubation at 37°C and 5% CO2 in a humidified incubator, AlamarBlue® (BUF012B, AbD Serotec) was added to each well according to the manufacturer’s directions and after 8 hours absorbance of the reduced dye was measured. In order to determine cell proliferation, the absorbance for mitogen stimulated T- and B- lymphocytes was calculated as a percentage of the absorbance of the unstimulated lymphocytes.
At 18 weeks of age all pullets were weighed and killed with intravenous pentobarbitone sodium. Specimens of small intestine (jejunum and ileum) were taken from eight pullets per treatment, opened lengthwise and stained with polychrome methylene blue (Amber Scientific, Australia)). Then the number of Peyer’s patches (Pp) ≥1mm2 were counted and the area of Pp and the small intestine areas were measured using AutoCAD Software (Autodesk AutoCAD). The total area of Pp in each pullet relative to the areas of their jejunum and ileum was calculated (relative Pp area). The experiment was approved by the La Trobe Animal Ethics Committee. Data were analysed using one-way analysis of variance (ANOVA, SPSS, 22, USA) and statistical significance between means of different treatment groups was compared by Tukey’s test at P<0.05.
Results and discussion
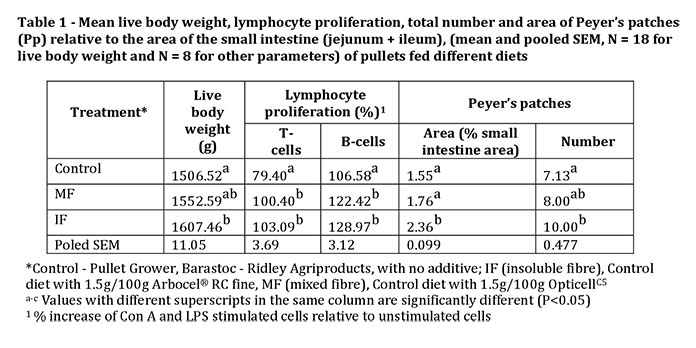
At 18 weeks of age, live body weight was significantly (P<0.05) increased in pullets receiving the IF dietary treatment compared to the control group but there was no difference between IF and MF pullets or between MF and Control pullets (Table 1). Proliferation of T-lymphocytes (Con A stimulated) and B-lymphocytes (LPS stimulated) of both IF and MF fed pullets were significantly (P<0.05) greater than those of the Control group (Table 1). The observed increase in lymphocyte proliferation indicates that both IF and MF have the potential to enhance the number of lymphocytes in brown pullets. Dietary fibre, both insoluble and soluble, from other sources has been shown to improve lymphocyte proliferation in poultry.
The area of Peyer’s patches (Pp) relative to area of small intestine was significantly (P<0.05) higher in pullets fed the diet supplemented with IF compared to the control and MF groups, and IF supplemented pullets had significantly (P<0.05) more Pp along the small intestine compared to the control group (Table 1). In mice, fibre in the form of fructo-oligosaccharides but not wheat bran has been shown to increase Pp number.
The positive effects on growth, Pp and lymphocyte proliferation may have been the result of an effect on reducing pathogens or changing microflora in the gut. It is also possible that improved activity of proteolytic digestive enzymes and digestibility of protein resulted in greater availability of protein to support immune system functions.
Conclusion
Addition of IF to the diets of pullets resulted in heavier body weights, higher T- and B- lymphocyte proliferation and increased numbers and sizes of Pp than observed in Control pullets: MF on the other hand only resulted in higher T- and B-lymphocyte proliferation. Therefore, increasing the concentration of IF in the diet of 10 week old pullets prior to point of lay, may be a useful alternative to antibiotics both as a growth promoter and to generate a greater number of B and T lymphocytes.
Acknowledgements: The Authors thank the Ministry of Higher Education and Scientific Research-Iraq for providing scholarships to Sherzad Mustafa Hussein and Johnny Shumuel Yokhana and the University of Duhok, Kurdistan Region, Iraq for giving them study leave. Authors also thank Rob Evans and the LARFT staff for help with care of pullets.
References are available on request
From the Proceedings of the Australian Poultry Science Symposium

















