
Based on tibia-ash content, equivalency between phytase enzymes with young turkeys appeared to be similar with young broilers. An extensive review about “microbial phytase in poultry nutrition”, including a long reference list, was made by Peter Selle and Velmurugu Ravindran (2007).
 In 2009, at this conference, Peter Woodward presented a review about “phosphorus and phytase” in turkey diets. In short, Phosphorus (P) is an essential nutrient for poultry, important for the development of strong healthy bones.
In 2009, at this conference, Peter Woodward presented a review about “phosphorus and phytase” in turkey diets. In short, Phosphorus (P) is an essential nutrient for poultry, important for the development of strong healthy bones.
Although it should not be forgotten that it is also important for meat production. P in plant material is bound to phytate molecules that are poorly digested by poultry. Inorganic phosphate sources are limited. Together with the ban in the European Union on the use of mammalian proteins in animal feed, like meat and bone meal which is rich in P and Ca, made P an expensive nutrient in poultry diets. Woodward calculated that cost of P represents more than 10% of the ingredient cost in a turkey starter diet.
The capacity of phytase, as exogenous feed enzyme to release phytate-bound P is evident. Effectively, phytase has become an alternative economical P source. Today, phytase enzymes are standard added to poultry diets to reduce dietary costs and the bird’s P excretion and thus sparing environmental pollution, but also inorganic P sources. Both references address that dietary supplemented phytase can also positively affect ileal absorption of amino acids and energy, but also that the P-release by phytase is not necessarily a fixed value for P but depends on many factors, like dietary phytate and calcium content. Most often, phytase producers are using the same P matrix values for broiler, turkey or duck diets. Compared to broilers, studies in which a P equivalence of phytase is determined for turkeys are scarce. This paper will compare the efficacy of two phytase sources in an experiment with young turkeys and with young broilers with a similar experimental set-up carried out at Schothorst Feed Research.
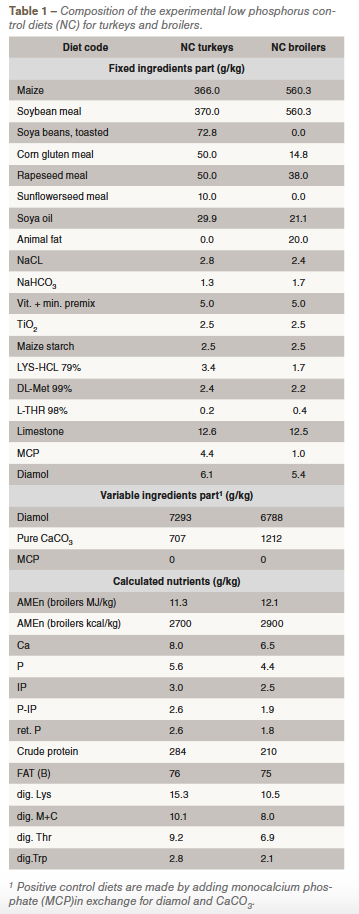
For young turkeys and young broilers each, a low P maize/soya based negative control diet (NC) was formulated (Table 1). Three positive control diets were made by adding 0.6, 1.2 and 1.8 g/kg P from monocalcium phosphate (MCP) by exchange with diamol and CaCO3. Compared to the NC diet the total Ca content in the PC diets was increased with 1.0 g/kg for the turkeys and 1.3 g/kg for the broilers (also as result of the exchange of MCP, diamol and CaCO3); different phytase test products were added at four graded dose levels to the NC diet resulting in 12 dietary treatments per animal species.
Test products were a Buttiauxella spp phytase expressed in Trichoderma reesei (BT) and an E. coli phytase expressed in Pichia pastoris (EP).
Both test products were analysed for standard phytase activity (FTU according AOAC) by LUFA (Oldenburg, Germany). Diets were cold pelleted and TiO2 was added as inert marker. Per animal species, each dietary treatments was tested with 6 replicate metabolic cages with 16 birds from 5-21 days of age. Feed and water were freely available. At day 21, content was collected from 21 cm ileum starting 1 cm proximal of the ileo-caecal junction. Pooled samples per cage were freeze dried and analysed for Ti and P to calculate apparent P absorption coefficient. Tibia bones were collected from 4 birds per cage and analysed for ash per kg fat free dry matter.
Response parameters were analysed for dietary treatment effect (ANOVA with Genstat program). Subsequently exponential dose-response curves were fitted per phytase product, response parameter and animal species. The experiments were approved by the animal ethic committee.
Results for apparent ileal P absorption coefficients (abs P) and tibia-ash content per dietary treatment are given for turkeys and for broilers in Table 2, expressed as percentage of the NC treatment (=100%).
Realized FTU dose levels were not identical per phytase and per animal species. This was caused by a dosing on expected FTU in the test products while products were analysed after making the experimental diets () showing a difference compared to expectations. For both animal species and both phytase enzymes, the lowest FTU inclusion level increased significantly ileal P absorption and tibia ash content. Although the highest FTU inclusion of EP was higher compared to BT phytase, the abs P with BT was yet significantly higher than with EP in either turkeys or broilers. With tibia-ash as response parameter this significant difference between the phytase products was present when 1155 FTU EP was compared with 1035 FTU BT in turkeys or 1505 FTU EP compared with 1046 FTU BT in broilers.
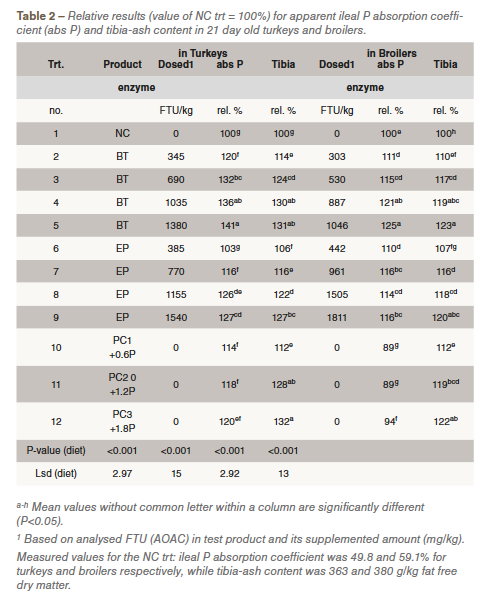
This means a clear higher significant efficacy per standard FTU of BT versus EP regardless of the animal species. This difference is also obviously seen when exponential dose-response curves are fitted. In Figures 1 and 2 the curves are given with tibia-ash as response parameter for turkeys and broilers, respectively. The curve equations were used to calculate the equivalence between the phytase products as given in Table 3. Based on tibia-ash content 500 EP was equivalent with 225 FTU BT in turkeys or with 260 FTU in broilers. This is rather similar between the animal species and around the factor 2.0 between BT vs EP. This factor is still present when determining the equivalence of EP with 500 FTU BT when a higher part of the curves has to be used. Although, relative response values by adding phytase appeared somewhat higher in turkeys, this means that the determined difference in efficacy between the two products in young turkeys was good comparable with young broilers.
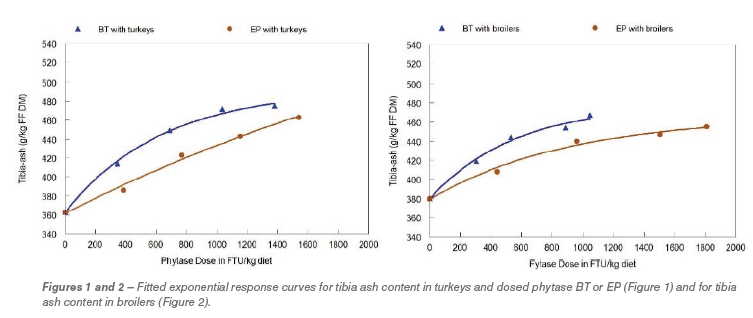
Evaluating ileal abs P the efficacy of BT was also higher compared to EP, but appeared larger with turkeys (factor ca. 2.5) and smaller with broilers (factor ca.1.5). The abs P is the situation at day 21 that might be (total) different to the situation at e.g. day 7 when supplying the experimental diets has just began. Because supply of dietary P is similar during the experimental period but the birds P requirement will decrease per kg diet when aging.
Another factor is the adaptation capacity to the NC diet, resulting in up-graded or up-regulated IP degradation in absence of phytase. For the NC diet, it was calculated that minimum IP degradation must have been 6% in turkeys but 26% in broilers. It may not be concluded that this capacity in broilers is larger than in turkeys, because it is likely related to the total P supply with the diet that is different between turkeys and broilers, while phytate-P (IP) level is also different. Tibia-ash content at day 21 is more a ‘weighed’ result of the experimental period and not just depended on the P absorption at day 21.
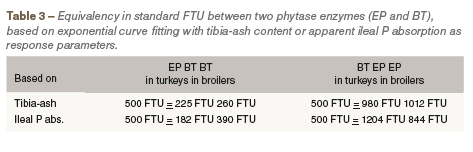 Therefore, tibia-ash content at day 21 gives most likely a better understanding of the phytase efficacy than the ileal abs P at day 21. Based on tibia-ash content and the response of the control diets it was calculated that 1.0 g MCP-P could be replaced by 674 FTU BT or 1215 FTU EP in turkeys, while in broilers this was calculated as 594 FTU BT or 1240 FTU EP. Of course, the difference between the phytase products corresponds with earlier given results. However, these values are not in line with values used in practice based on many more trials where 500 FTU E.coli phytase can replace at least 1.0 g MCP-P.
Therefore, tibia-ash content at day 21 gives most likely a better understanding of the phytase efficacy than the ileal abs P at day 21. Based on tibia-ash content and the response of the control diets it was calculated that 1.0 g MCP-P could be replaced by 674 FTU BT or 1215 FTU EP in turkeys, while in broilers this was calculated as 594 FTU BT or 1240 FTU EP. Of course, the difference between the phytase products corresponds with earlier given results. However, these values are not in line with values used in practice based on many more trials where 500 FTU E.coli phytase can replace at least 1.0 g MCP-P.
This is depending on many factors which will not further be addressed in this paper but does show the complexity of determining absolute P matrix values.
Conclusions
Based on tibia-ash content the difference in efficacy in young turkeys was comparable with young broilers. Determination of absolute P replacement values of phytase enzymes is a complex matter regardless of the animal species.
Acknowledgment
The turkey and broiler experiment were sponsored by Danisco Animal Nutrition.
References
P.H. Selle and V. Ravindran, 2007. Microbial phytase in poultry nutrition. Animal Feed Science and Technology 135 (2007) 1-41.
Peter Woodward, 2009. Phosphorus and phytase for turkeys. Proceeding of the 3rd Turkey Science and Production Conference, Macclesfield, UK.
From the Proceedings of the Turkey Science and Production Conference

















