The effectiveness of phytase is usually determined under phosphorus or both calcium and phosphorus deficient specifications. Phytase efficacy in terms of increasing available or digestible P and/or Ca are often determined without considering the impact of other dietary factors. This study was designed to determine the impacts of dietary calcium (Ca), phytate phosphorus and non-phytate phosphorus on myo-inositol hexaphosphate (IP6) degradation, in the presence and absence of phytase in broiler birds. Ravindran et al. (1995) reported that phytase was less effective in improving P utilization when birds were offered diets with adequate non- phytate phosphorus as compared to those offered non-phytate phosphorus deficient diets. However, the implications of the potential interactions between calcium, non-phytate phosphorus, phytate phosphorus and phytase concentrations on phytate utilization are not clearly understood.
Among the majority of the current commercial phytases, pH optima for maximum activity is between 3 and 5, suggesting that the upper segments of the gastrointestinal tract (GIT) namely the proventriculus (Prov), gizzard (Giz) and possibly the crop are the primary active sites for phytase activity. In addition, in vitro studies have demonstrated that phytate precipitates with Ca at a pH higher than 4, resulting in decreased de-phosphorylation of phytate by phytase. This highlights the importance of phytate degradation in the more acidic upper portions of the GIT in the presence of exogenous phytase and the potential implications in phytate phosphorus digestion and absorption in the lower gut. Although several studies published recently investigated IP6 degradation in different segments of the GIT, dietary impacts of calcium, phytate and non-phytate phosphorus concentrations are rarely mentioned. Therefore, the objectives of this study were to determine the impacts of dietary calcium, phytate and non-phytate phosphorus concentrations in the presence and absence of phytase on 1) IP6 concentrations in the crop, Prov plus Giz, and ileum; and 2) the apparent IP6 digestibility up to the distal ileum.
Materials and methods
The experiment was conducted twice (block) in time with three replicates (10 birds/replicate) of each treatment represented in each block (n=6). Birds were raised in floor pens and fed a commercial starter diet formulated to meet or exceed all NRC (1994) recommendations until 10 days of age. On day 11, birds were individually weighed, grouped to minimize within and between group weight variations and placed into battery pens which were preassigned to treatments. On day 13, all birds in a pen were euthanized by cervical dislocation and contents from crop, Prov plus Giz, and distal ileum were collected from each bird. Two corn and SBM mash basal diets with either low or high phytate phosphorus were formulated based on analysed ingredient compositions, mixed and analysed for dry matter, macro-minerals, protein, ether extract and amino acids.
Meat meal (5.7%) and rice bran (6.0%) were included in the low and high phytate phosphorus basal diets, respectively, to achieve the desired differences in phytate phosphorus concentration while maintaining similar concentrations of other nutrients. Based on analysed calcium and phosphorus concentrations in the basal diets, pre-analysed limestone and mono-calcium phosphate were added to achieve desired calcium and non-phytate phosphorus concentrations in treatment diets. Basal diets containing either low or high phytate phosphorus were included at 96.7% in the final diets, titanium dioxide was added at 0.3% as the inert marker and silicon dioxide was used as a filler to achieve 100%. The experiment was a 2*2*2*3 randomized block design with two calcium (0.7 and 1.0%), two phytate phosphorus (0.23 and 0.34%), two non-phytate phosphorus (0.28 and 0.45%) and three phytase (0, 500 and 1000 FTU/kg) resulting in a total of 24 treatments. For each diet without phytase, a 6-phytase (Buttiauxella sp.) was added on top, at either 500 or 1000 FTU/kg to one of the three lots of the treatment and mixed. The starter and treatment diets were fed as mash throughout the trial. Data were analysed as a randomized complete block design using SAS. Tukey’s test adjustment was applied in all pair-wise comparisons. Significance was declared at P < 0.05.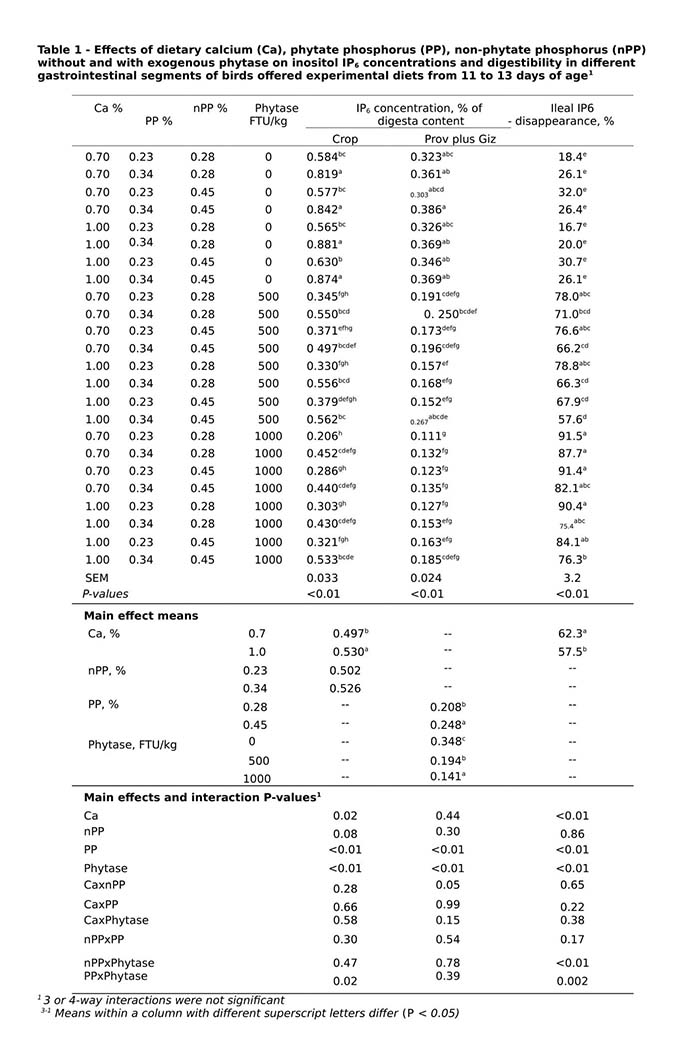
Results and discussion
Decreased IP6 concentrations in all three segments of GIT and increased ileal IP6 disappearance were seen with phytase inclusion regardless of diet Ca, non-phytate phosphorus or phytate phosphorus concentrations (Table 1; P<0.05). Increasing phytase inclusions improved IP6 degradation rate, addition of 500 and 1000 FTU/kg phytase degraded up to 79% (62% above control) and 91.5% (73% above control) IP6 respectively at the ileal level. Despite the interaction between phytate phosphorus and phytase (Table 1, P<0.05), crop IP6 concentration was significantly reduced by an average of 53 and 46% with 1000 FTU phytase/kg at 0.23 and 0.34% phytate phosphorus, respectively (P < 0.05). Dietary Ca had a significant impact on IP6 degradation. Across all non-phytate phosphorus, phytate phosphorus and phytase concentrations, increasing Ca from 0.7 to 1.0% resulted in 7% increase in crop IP6 concentration (0.530 vs. 0.497; P<0.05). Crop IP6 concentration was not affected by non-phytate phosphorus concentrations (P>0.05).
Prov plus Giz IP6 concentration was affected by non-phytate phosphorus, phytate phosphorus and phytase. There was an interaction between Ca and non-phytate phosphorus on Prov plus Giz IP6 concentration, where 14% higher IP6 concentration was seen in birds fed 0.45% than 0.28% non-phytate phosphorus diets at 1.0% Ca (P<0.05). Ileal IP6 disappearance was 9% lower when diet non-phytate phosphorus increased from 0.28 to 0.45% with 500 FTU phytase/kg inclusion, but this reduction in IP6 disappearance was not seen when phytase dose was increased to 1000 FTU /kg.
Zeller et al. (2015) also reported a similar interaction between added non-phytate phosphorus and phytase inclusion level, where adding non- phytate phosphorus from mono-calcium phosphate (0.09% non-phytate phosphorus) in the presence of 500 FTU of an E.coli phytase/kg resulted in a lesser degree of IP6 degradation in the ileum but added non-phytate phosphorus had no impact on IP6 degradation when phytase was increased to 12500 FTU/kg. Results from the present study and that of Zeller et al. (2015) support the concept that the impact of non-phytate phosphorus on phytate degradation is dependent on phytase inclusion levels.
In conclusion, Buttiauxella phytase at 500 and 1000 FTU/kg improved IP6 degradation by up to 62 and 73 percentage points above respective controls, indicating a high phytase efficacy. IP6 degradation rate was associated with dietary factors, reducing dietary non-phytate phosphorus and Ca concentrations can have a positive effect on IP6 degradation in the presence of exogenous phytase.
References are available on request
From the Proceedings of the Australian Poultry Science Symposium