
Butyrate is a molecule that is extensively studied as a feed additive to improve gut health and animal performance. It also has been described to reduce the expression of colonizing genes in Salmonella. When applied in an in vivo Salmonella challenge model, Van Immerseel and co-workers (2005) demonstrated that a fat matrix-protected butyrate, but not an unprotected butyrate, was able to reduce caecal Salmonella load and shedding.
Hypothesising that the difference in these effects was due to the distinct butyrate release profiles of both products in the gastro-intestinal tract (GIT) tract of animals, investigations were conducted into the relationships between butyrate formulations, the butyrate delivery characteristics in vitro models of different GIT segments, the effect on caecal butyrate concentration in vivo, and the capacity of the different formulations to confer resistance to caecal Salmonella colonization in vivo.
Method
Different formulations of butyrate products, each with a distinct expected release profile, were investigated: uncoated sodium butyrate (‘SB’, absorption in proximal GIT), tributyrin (‘TB’, small intestine), 30% fat-protected butyrateand two prototypes with supposed superior hindgut release, with butyrate embedded in a micro-crystalline wax matrix: one contained 30% butyrate and 70% wax (‘Wax’), while the other was composed of 30% butyrate, 60% wax and 10% soluble potato starch (‘Wax+’). Addition of starch to the wax has a disintegrative effect, making the matrix less resistant, thereby influencing the release rate of butyrate.
After differences in butyrate release characteristics were confirmed in the in vitro models (data not shown), two in vivo trials were conducted. The first trial was set up to screen all the butyrate formulations. One pen of 20 animals was allocated to each of the 6 dietary treatments. The second trial was used to confirm the performance effects of the products that yielded the best results in the first trial. Two pens of 20 animals were allocated to 3 treatments: negative control, FPB, and Wax. These feed additives were included in the diets at 3 g of butyrate per kg of feed.
In each experiment, 17-day-old broilers where orally infected with 105 CFU of S. Enteritidis phage type 4 strain 147. Four days post-infection, birds were euthanised and caecal content was analysed for short-chain fatty acid (SCFA) concentrations and for Salmonellaload. For SCFA quantification, acetate, propionate and butyrate were extracted with diethyl-ether and analyzed on a gas chromatograph coupled with a flame-ionization detector. Bacterial analysis was performed by homogenising the caecal samples and plating a ten-fold dilution series on streptomycin-supplemented XLD plates.
After incubation overnight at 37 °C, the number of colonies was determined and numbers of CFU/g calculated. Samples that were negative after direct plating were enriched in BGT broth at 37 °C overnight and plated. When found to be positive after this step, these samples were presumed to have 83 CFU/g (detection limit of direct plating).
Caecal microbial analysis was performed via 16S rRNA sequencing using MiSeq v2 technology from Illumina. Caecal content was collected from the 21-day-old chickens in the first trial 4 days after Salmonella infection. DNA was extracted from the caecal content, and relative abundances were determined.
Data from the first trial were analyzed with a Kruskal–Wallis test. All pairwise differences between the treatments were assessed using Behrens Fisher tests. The data from the second trial and the SCFA measurements were assessed by one-way ANOVA. To determine statistical differences in relative abundances of the bacterial families, non-parametric Kruskal–Wallis test was used.
Results
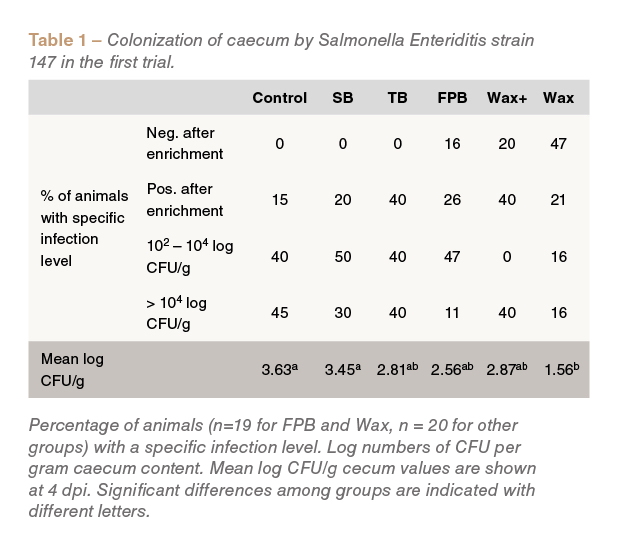
In the first trial, the lowest mean caecal Salmonellacounts were observed in birds fed the Wax treatment (P<0.05 compared to the control) (Table 1).
Birds fed Wax, Wax+ and FPB were also found to be negative for Salmonella infection strain, unlike those fed the other dietary treatments (Table 1).
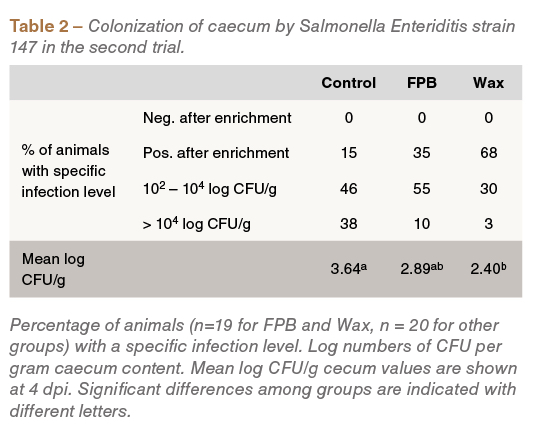
Similar observations were detected in the second experiment: compared to the control group, Wax-fed birds had a lower mean Salmonella count (P<0.05) (Table 2).
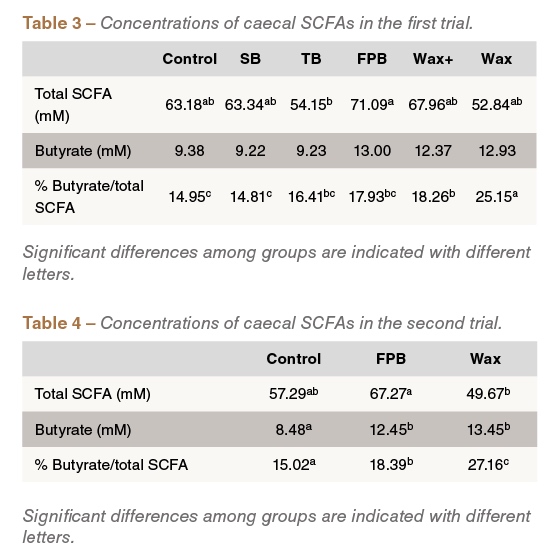
In the first experiment, total SCFA and butyrate concentration was numerically higher in birds fed the FPB, Wax+ and Wax treatments compared to those fed the control treatment. The relative abundance of butyrate, as percentage of the total SCFA concentration, was significantly higher in the Wax-fed group (P<0.05), compared to the control (Table3). SCFA analysis in the second trial yielded similar findings: both the absolute and relative butyrate concentration were higher in the FPB and Wax-fed groups compared to the control fed group (Table 4).
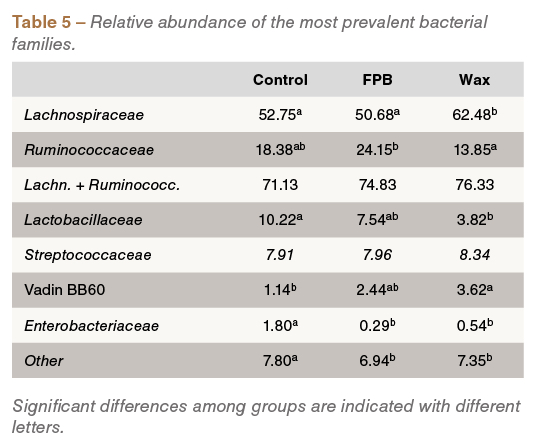
Interestingly, supplementation of wax- and fat-coated butyrate was linked to changes in caecal microbial composition; for example, an increased prevalence of butyrate-producing Lachnospiraceae and Ruminococcaceae, and a reduced abundance of Enterobacteriaceae and Lactobacillaceae, was detected in birds fed these treatments (Table 5).
Discussion
These results demonstrate that there is a correlation between delay in butyrate release, increased hindgut butyrate concentrations and reduction in caecal Salmonella numbers. More specifically, the data suggest that dietary supplemented butyrate needs to be protected in order to increase caecal butyrate concentrations and protect broilers against SalmonellaEnteritidis.
Of note, microbial analysis suggests that at least part of the elevated caecal butyrate concentration in the protected butyrate-fed broilers is the result of shifts in caecal microbial composition. Other studies have been published indicating that butyrate supplementation can influence caecal microbiota composition of challenged birds in a way that seems beneficial for health and growth. However, the exact mechanisms underlying these effects remain to be elucidated.
In addition, this study suggests that novel butyrate formulations can be developed with improved efficacy against zoonotic pathogens residing in the hindgut of broilers.
References are available on request
From the Proceedings of the Australian Poultry Science Association – 2021

















