
In the current context of reduction of the use of antibiotics in the livestock sector and with the intention of providing its own contribution to this process, Albors carried out studies in the direction to find effective and sustainable solutions, in collaboration with important research centers (CNR and University of Milan). The aim of the project was the development of an innovative product with natural antibacterial and immune-stimulating properties.
Antibiotic resistance is a hot topic and it has generated a worldwide alarm since several years. The World Health Organization (WHO) and the European community seriously approached this issue by indicating several specific measures with the scope to stop the spread of antimicrobial resistance through the sensible use of antibiotics in humans as well as in animals. The livestock sector is learning how to minimize the use of such molecules, thanks to targeted measures that concern various aspects of farming: feed quality, biosecurity, management, genetics, animal welfare and alternative ingredients capable of reinforcing the immune system and preventing diseases.
In this framework, Albors decided to invest in the research of new substances with bactericidal and/or immune-stimulating activity, with the intention to develop an innovative product to be used in animal nutrition.
The first stage consisted on the research of what was available on scientific literature as potentially interesting natural molecules to be included in the formulation. The first investigation of the bibliography led to the selection of 6 molecules or natural compounds commercially available in the market, that have not been yet studied and tested thoroughly in animal husbandry. All the available scientific evidences related to these compounds were collected, in order to study their spectrum of activity and the dosage at which they are currently used in different applications.
Thanks to the collaboration with the Institute of Food Production Sciences of the National Research Council (CNR-ISPA) in Milan, the molecules and selected compounds were tested individually in vitro in order to evaluate their action against bacterial strains of zootechnical interest such as: Clostridium perfringens BAC LO SMN, Escherichia coli BAC RE RB 49, and Salmonella typhimurium BAC RE RB 1743, acquired from the collection of the “Experimental Zooprophylactic Institute of Lombardy and Emilia Romagna”, Staphylococcus aureus ATCC 19095 (American Type Culture Collection ) and two strains of Streptococcus uberis isolated from bovine mastitis (705 and 707), belonging to the collection of the Department of Veterinary Medicine of the University of Milan.
The efficacy of the individual ingredients was determined by evaluating the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC), according to the method described by Wiegand et al., 2008. Based on the reports, it was decided to focus the research only on 2 out of the 6 initially selected substances: Cinnamomum verum extract and a by-product of the fermentation of Aspergillus niger, which showed a broad-spectrum antibacterial activity against all the target microorganisms, both Gram positive and Gram negative (Table 1). Specifically, the Cinnamomum verum extract showed its effectiveness in a concentration range of 250 and 4,000 ppm, while that of the by-product of A. niger fermentation between 25 and 50 ppm.
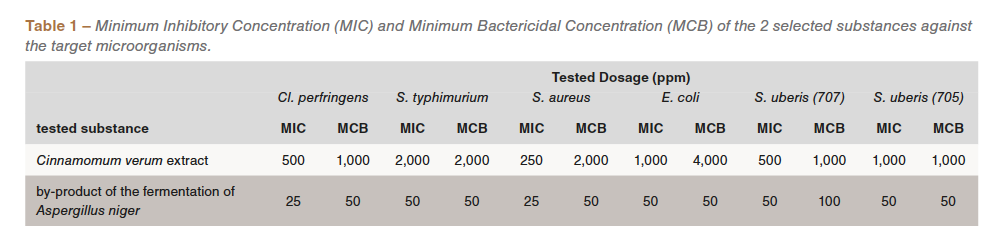
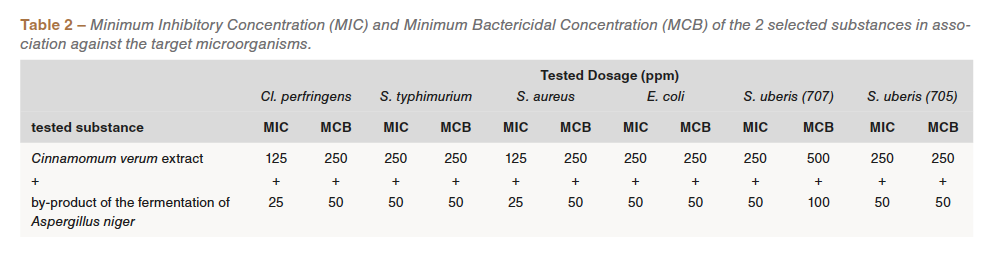
Subsequently, the two substances were studied in combination to verify the synergistic effects. Keeping the dosage of the A. niger fermentation by-product unchanged, it was in fact possible to inhibit the development of target microorganisms by reducing the concentration of Cinnamomum verum extract in a range of 125 and 500 ppm (Table 2).
As part of the collaboration with the CNR-ISPA it was also possible to verify the action of the two selected substances, single and in association, against some “good” useful commensal bacteria, those microorganisms that guarantee an important gut microflora equilibrium in favor of the intestinal health. For this purpose, the following bacterial strains were taken into considerations: Lactobacillus casei VC199, Enterococcus faecium VC223 and Streptococcus thermophilus SE95, belonging to the collection of CNR ISPA Milano. As previously described, also for these bacterial species the same in vitro determination of the MIC and of the MBC was performed (Table 3).
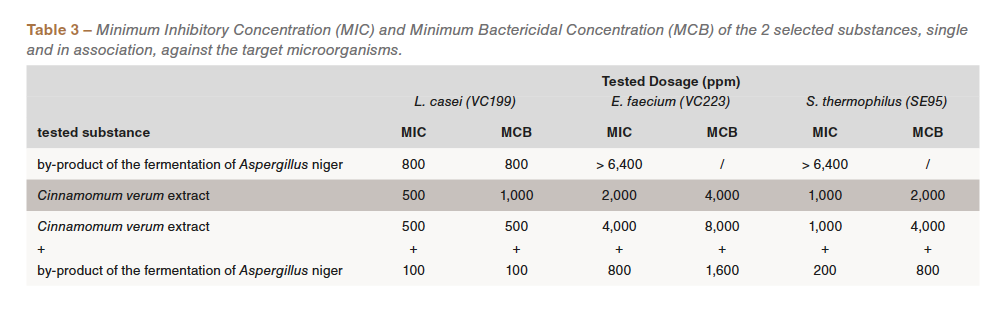
These tests showed that the two substances could damage the commensal bacterial population only at high concentrations, while a reduced dosage is sufficient to inhibit the growth of the tested pathogens. This phenomenon can be used to promote the growth of beneficial bacteria in the intestine, limiting the colonization of harmful microorganisms.
After the tests on the antibacterial activity, the project continued including the two components of greatest interest within a more complete formulation, where the antibacterial activity could also be associated with an immune-stimulating and antioxidant effect on the organism. In this regard, other substances of natural origin were considered, a form of oligosaccharides derived from dairy milk with prebiotic activity and tocopherols with antioxidant action, which were then added to the previous combination of ingredients.
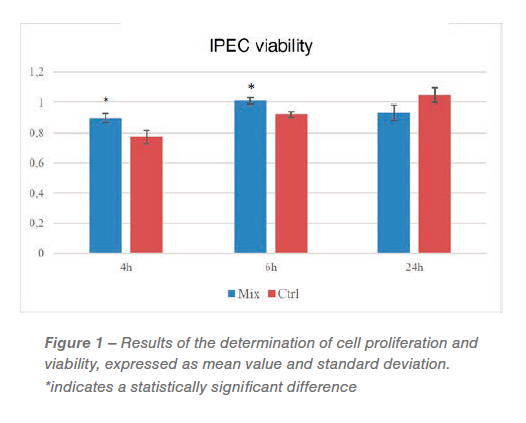
The new formulation, named albonat®, was tested to evaluate its cytotoxic activity on the intestinal epithelial cell line IPEC-J2 at the University of Milan (Department of Veterinary Medicine), to ensure that the combination of ingredients does not cause adverse effects on the intestinal mucosa once ingested by the animal. For this purpose, the cellular line was placed in contact with albonat® (Mix) and a neutral control solution and then examined at intervals of 4, 6 and 24 hours, evaluating the morphology of the cells using an inverted microscope and performing a test for the determination of cell proliferation and viability, by MTT (Cell Proliferation Kit I, Sigma).
The results are visible in Figure 1, the cells in contact with albonat® (Mix) showed a significantly higher viability at 4 and 6 hours compared to the control (P=0.01 and P<0.003 respectively). At 24 hours, however, the treated cells showed a slightly lower viability, but without any significant difference. This last data is however not important, since the formulation does not remain continuously in contact with the epithelium for such a long period of time after the ingestion takes place.
On the basis of the above-described scientific tests, Albors implemented the technological and industrial development of albonat®, as a complementary feed containing the active ingredients included in the research. This product was afterwards tested in field trials on live animals with positive results. Two versions of albonat® are commercially available, the powder form to be used in drinking water (albonat® WS) and the heat resistant micro-granule form to be included in the pelleted feed (albonat® coated).

For more information please contact:
Gianfranco Russo Responsabile tecnico commerciale, ALBORS Srl, Milano, Italia
www.albors.it – commerciale@albors.it

















