
Mycoplasma are the smallest free-living organisms both in size and number of genes, and unlike many other bacteria, they do not have a cell wall. There are two Mycoplasma species, Mycoplasma gallisepticum (MG) and Mycoplasma synoviae (MS), which cause disease in chickens. MG is generally the more pathogenic species and causes major economic losses. MS is more widespread and is a common pathogen on multi-aged commercial layer farms.
Introduction
Mycoplasma synoviae (MS) strains vary widely in their ability to cause disease, with many strains appearing mild. More pathogenic MS strains can produce significant respiratory disease, egg production loss, and joint infections in susceptible birds.
MS may not always be the primary pathogen and often occurs as a mixed infection with other respiratory pathogens, particularly Newcastle disease virus (NDV) and infectious bronchitis virus (IBV). Chronic respiratory disease from these mixed infections can become significant, particularly in adverse environmental conditions like high ammonia, cold temperatures and dust. MS-infected birds may be more reactive to live NDV and IBV vaccinations. MS has been implicated in the pathogenesis of E. coli-induced egg yolk peritonitis in layers.
Clinical signs
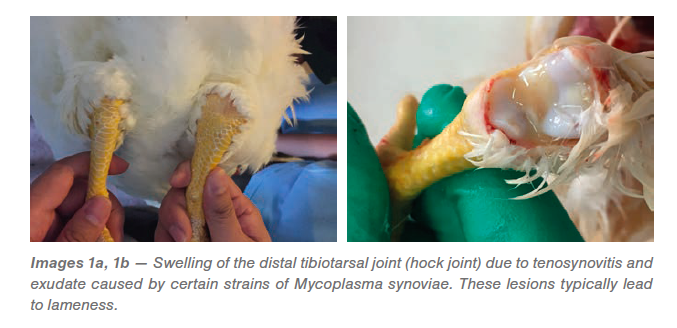
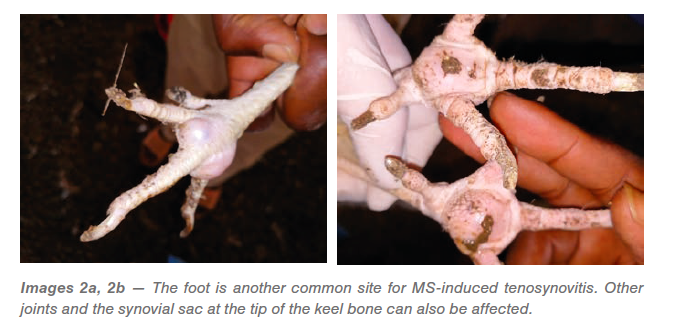
Mycoplasma synoviae in chickens commonly occurs as an infection of the upper respiratory tract and may present with slight rales (respiratory sounds) but is typically unnoticed (subclinical). With some strains of MS, there may be a progression from the acute to the chronic phase when the infection spreads to the joints (Images 1a, 1b). Colonization of the joint tissue produces inflammation of the synovial membranes and tendons (exudative tenosynovitis), eventually resulting in lameness. Hock (tibial metatarsal) joints and foot pads are the primary areas involved, but other joints and the keel bone bursa may also be affected. This form can be seen in flocks as young as 4 weeks, but typically presents shortly after transfer of mature pullets to the laying farm (Images 2a, 2b).
Effect on egg production
If the flock is exposed to MS during the rearing period, usually there is little effect on egg production. Flocks challenged during the laying period may experience a reduction in egg production and less collectable eggs. The egg production curve in a MS-positive flock can have a “rollercoaster” appearance, especially in flocks on a program of periodic antibiotic feed treatment. Tenosynovitis resulting in lameness can further negatively affect egg production due to poor mobility to feed, water, and nests.
Effect on Eggshell Quality
Recently, MS strains with oviduct tropism have emerged in commercial layers. Flocks infected with these strains of MS are observed with increased numbers of broken and cracked eggs. A characteristic eggshell defect on the apical end (pointed end) of the egg can be observed (Images 3a, 3b). The eggshell surface 2 cm from the apex of the egg is rough, with thinning and translucency described as glass eggs. Scanning electron microscopy shows that these eggshells lack the mammillary knob layer and part of the palisade layer.

Transmission
Many avian species can carry and propagate MS, including chickens, turkeys, and most wild birds, especially Passeriformes (perching and songbirds). Horizontal transmission of MS between birds occurs by direct contact with respiratory secretions, resulting in slow spread through the flock. The rate of spread through the flock is 1–4 weeks and largely dependent on the pathogenicity of the infectious strain, housing type, and environmental conditions. In general, mycoplasmas have a slow progression through a facility, but eventually will infect all birds in the flock. Not all infected birds will develop clinical signs of MS. Infected birds remain potential shedders, and even with antibiotic treatment the flock will likely remain infected and be lifelong carriers.
Vertical transmission is an important factor in the spread of MS. When a parent flock becomes infected, the highest rate of shedding occurs 4–6 weeks post infection. Thereafter, the rate of vertical transmission to chicks usually decreases and may become intermittent, coinciding with periods of stress.
Using hatching eggs from MS-positive breeder flocks is highly discouraged. If MS-positive parent flocks must be utilized, then the progeny should be hatched separately, and the destination farm isolated. Antibiotic treatment of the chicks for the first 2 weeks of life may also be indicated and increased monitoring is helpful.
MS can persist and remain infective for 2–3 days on feathers and various materials such as cotton, rubber, and wood. Mycoplasma can be carried in the human nose. Its small size and weight allow it to be carried up to 8 km (5 mi) in the air on dust or dander particles. Work done on synthetic hair showed survival times of up to 9 days. Mycoplasmas can survive longer in water, soil, and in egg material.
Incubation period
Incubation following contact exposure is typically 11–21 days. Chicks that are infected by vertical transmission typically show clinical signs of MS within a few days after hatching.
Diagnosis
Diagnosis of MS is based on observing clinical signs of respiratory disease and lameness. The clinical picture of the respiratory disease produced by MS is like that of other respiratory pathogens. The joint swelling and synovitis lesions produced by MS are like those produced by other bacteria like Staphylococcus. Apical eggshell abnormalities, when present, are suggestive of MS involvement. Finally, laboratory confirmation of MS infection is required for a definitive diagnosis.
Laboratory
The focus on testing for Mycoplasma synoviae is different depending on the type of flock. For commercial birds, the goals are to see if vaccination was successful, when the flock seroconverts naturally, or to see if a negative flock status is maintained. For all of these flocks, serological tests are more common to use. In parent stock flocks, because the goal is to remain negative, more sensitive and specific tests are recommended. As a result, PCR is becoming the favored test, as the time for detection is more rapid and more accurate.
The common serological tests include serum plate agglutination test (SPA, RPA), hemagglutination inhibition (HI), and enzyme linked immunosorbent assay (ELISA), all of which measure MS-specific antibodies of different types. SPA detects IgM antibody, found 3–5 days after infection and can persist for up to 80 days.
HI and ELISA detect IgG antibody, typically found 7–10 days after infection, which can persist for up to 6 months. All serological tests for MS may show a low level of false positive results. False positives are most commonly observed in young chicks and birds vaccinated with an oil-emulsion 2–4 weeks prior to the serum test. Serological tests should therefore only be utilized for screening purposes, and positive results must be followed by isolation or PCR testing for confirmation.
Polymerase chain reaction (PCR) is becoming the preferred method to confirm MS infection in a flock. The test detects MS-specific DNA, which implies the MS organism was in the bird at the time of sampling. MS-specific PCR tests have high sensitivity and specificity. The test only takes a few hours to obtain a result and will detect MS infection before serological tests are able to pick up a positive. Due to this, many parent stock farms are now using PCR sampling for screening purposes. For this it is important to sample a minimum of 25 birds. The best samples should be taken from the bird’s choanal cleft and/or synovial membranes. An additional benefit to PCR testing is that specific DNA probes have been developed to differentiate MS field and vaccine strains.
Culturing MS is most successful from acutely affected birds, becoming more difficult as the infection progresses. Samples include affected respiratory organs (trachea, air sacs, lungs, and sinuses). Should the birds display lameness, the affected synovial membranes and any exudate can be sampled.
Isolation of mycoplasma requires special culture media and technique taking several days for a result.
Immunofluorescence tests on mycoplasma colonies is a rapid and reliable method of identification of MS.
Treatment
In vitro antibiotic testing has shown MS to be sensitive to several antibiotics including aivlosin, tylosin, tiamulin, chlortetracycline, oxytetracycline, lincomycin, kitasamycin, enrofloxacin and danofloxacin. Repeated field use of any antibiotic increases the chance for bacteria to develop resistance; therefore, it is important to determine the sensitivity of the isolate(s) to several antibiotics before making an antibiotic selection for treatment. Antibiotic selection should always be made based upon local regulations and under the guidance of a veterinarian.
Treatment of existing tenosynovitis is often unrewarding, as lameness occurs from irreversible scarring and inflammation of the synovial tissues and tendons. High doses of antibiotic are often required for the flock to respond on a meaningful level. Short, high-dose intervals of medication are preferred over long-term medication, as this increases the risk of developing resistance.
There is some field evidence that positive flocks can be treated into a fully negative status with high doses of continuous antibiotics together with strict biosecurity practices.
Effective biosecurity measures
An analysis of the potential risks for introduction and spread of the disease should be carried out on each farm and procedures put in place to minimize this risk.
Some examples would include:
- avoid multi-aged laying farms where older infected flocks can infect younger flocks. Remember that previously infected flocks will intermittently shed MS for their entire life;
- distance from neighboring poultry should be maximized;
- no poultry other than the current flock(s) should be allowed on the premises and employees should not have any interaction with poultry when not at work;
- interaction with neighboring poultry should be prevented;
- disinfect vehicles and equipment at point of entry of farm and/or site;
- personnel use of shower facilities for staff;
- provide personal protective clothing and footwear which is cleaned and remains on farm;
- use hygiene practices like washing and disinfection of hands between houses/barns;
- control pests (wild birds and rodents) and prevent access to the poultry facilities;
- dispose of mortality in an effective, timely manner;
- use of solid floors and walls make cleaning and disinfection easier and more effective.
Vaccination
Live MS vaccine reduces the shedding of pathogenic MS, which in turn decreases the level of environmental contamination.
The antibodies developed in response to the MS vaccine appear to play a critical role in preventing infection. If there is an infection, the antibodies may help aid the recovery from a MS field challenge. Vaccinated flocks that encounter MS field strain show lower levels of infection (PCR field strain positives) compared to naïve flocks. In addition, these vaccinated flocks have shown the ability to recover and even eliminate the field MS strain some weeks post challenge with the aid of medication, whereas the naïve flocks remain MS field positive.
For live vaccination to be effective, the birds must receive the vaccination before exposure to field strains. If early exposure is suspected or anticipated, it is advisable to protect the flock with prophylactic antibiotic treatment until the flock can be vaccinated.
Keep in mind that antibiotics should be withdrawn for an appropriate period (at least 7 days) before attempting to use live MS vaccine.
The MS-H vaccine requires eyedrop vaccination for best results. The vaccine is not stable at room or refrigeration temperatures and must be frozen at <-70 °C.
Vaccination is often avoided by commercial producers because the vaccine has special requirements for storage and preparation. While these vaccine traits pose a challenge, with proper preparation, the process can be very successful.
The additional cost of vaccination should be considered, but costs to medicate an infected flock and the potential losses in production often far outweigh the cost of vaccination.


















